Abstract
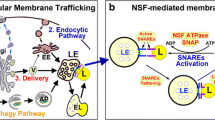
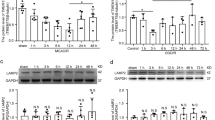
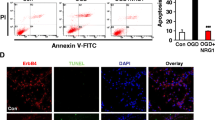
Neurons have extraordinary large cell membrane surface area, thus requiring extremely high levels of intracellular membrane-trafficking activities. Consequently, defects in the membrane-trafficking activities preferentially affect neurons. A critical molecule for controlling the membrane-trafficking activities is the N-ethylmaleimide-sensitive factor (NSF) ATPase. This study is to investigate the cascade of events of NSF ATPase inactivation, resulting in a massive buildup of late endosomes (LEs) and fatal release of cathepsin B (CTSB) after transient cerebral ischemia using the 2-vessel occlusion with hypotension (2VO+Hypotension) global brain ischemia model. Rats were subjected to 20 min of transient cerebral ischemia followed by 0.5, 4, 24, and 72 h of reperfusion. Neuronal histopathology and ultrastructure were examined by the light and electron microscopy, respectively. Western blotting and confocal microscopy were utilized for analyzing the levels, redistribution, and co-localization of Golgi apparatus and endosome or lysosome markers. Transient cerebral ischemia leads to delayed neuronal death that occurs at 48–72 h of reperfusion mainly in hippocampal CA1 and neocortical (Cx) layers 3 and 5 pyramidal neurons. During the delayed period, NSF ATPase is irreversibly trapped into inactive protein aggregates selectively in post-ischemic neurons destined to die. NSF inactivation leads to a massive buildup of Golgi fragments, transport vesicles (TVs) and late endosomes (LEs), and release of the 33 kDa LE type of CTSB, which is followed by delayed neuronal death after transient cerebral ischemia. The results support a novel hypothesis that transient cerebral ischemia leads to NSF inactivation, resulting in a cascade of events of fatal release of CTSB and delayed neuronal death after transient cerebral ischemia.







Similar content being viewed by others
Change history
23 May 2018
The author name “Dr. Jiang Wu” needed to be added as the 3rd author. The author institutional affiliations are correspondingly adjusted. The authors regret these errors.
The original article has been corrected.
Abbreviations
- NSF:
-
N-ethylmaleimide sensitive factor ATPase
- SNAREs:
-
Soluble NSF attachment protein receptors
- SNAP:
-
Soluble NSF attachment protein
- CTSB:
-
Cathepsin B
- TVs:
-
Transport vesicles
- LE:
-
Late endosome
- EL:
-
Endolysosome
- L:
-
Lysosome
- MOMP:
-
Mitochondrial outer membrane permeabilization
- IRI:
-
Ischemia-reperfusion injury
- DG:
-
Dentate gyrus
- EM:
-
Electron microscopy
- Vti1b:
-
Vesicle transport through interaction with t-SNAREs homolog 1B
- TGN38:
-
Trans-Golgi network membrane protein 38 kDa
References
Smith ML, Auer RN, Siesjo BK. The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol (Berl). 1984;64:319–32.
Wang W, Redecker C, Bidmon HJ, Witte OW. Delayed neuronal death and damage of GDNF family receptors in CA1 following focal cerebral ischemia. Brain Res. 2004;1023:92–101.
Horn M, Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol. 1992;85:79–87.
Kirino T, Sano K. Fine structural nature of delayed neuronal death following ischemia in the gerbil hippocampus. Acta Neuropathol. 1984;62:209–18.
Hu BR, Martone ME, Jones YZ, Liu CL. Protein aggregation after transient cerebral ischemia. The Journal of Neuroscience. 2000;20:3191–1999.
Hu BR, Janelidze S, Ginsberg MD, Busto R, Perez-Pinzon M, Sick TJ, et al. Protein aggregation after focal brain ischemia and reperfusion. J Cereb Blood Flow Metab. 2001;21:865–75.
Liu CL, Ge P, Zhang F, Hu BR. Co-translational protein aggregation after transient cerebral ischemia. Neurosci. 2005;134:1273–84.
Zhang F, Liu CL, Hu BR. Irreversible aggregation of protein synthesis machinery after focal brain ischemia. J Neurochem. 2005;98:102–12.
Wang D, Chan CC, Cherry S, Hiesinger PR. Membrane trafficking in neuronal maintenance and degeneration. Cell Mol Life Sci. 2013;70(16):2919–34.
Yuan D, Liu C, Hu B. Dysfunction of membrane trafficking leads to CTSB release and brain ischemia-reperfusion injury. Transl Stroke Res. 2017; in press.
Morgan A, Burgoyne RD. Is NSF a fusion protein? Trends Cell Biol. 1995;5:335–9.
Mohtashami M, Stewart BA, Boulianne GL, Trimble WS. Analysis of the mutant Drosophila N-ethylmaleimide sensitive fusion-1 protein in comatose reveals molecular correlates of the behaviouralparalysis. J Neurochem. 2001;77:1407–17.
Robinson LJ, Aniento F, Gruenberg J. NSF is required for transport from early to late endosomes. J Cell Sci. 1997;110:2079–87.
Dalal S, Rosser MFN, Cyr DM, Hanson PI. Distinct Roles for the AAA ATPases NSF and p97 in the Secretory Pathway. Glick B, ed. Mol Biol Cell. 2004;15(2):637–48.
Offenhauser C, Lei N, Roy S, Collins BM, Stow JL, Murray RZ. Syntaxin 11 binds Vti1b and regulates late endosome to lysosome fusion in macrophages. Traffic. 2011;12:762–73.
Luzio JP, Gray SR, Bright NA. Endosome-lysosome fusion. Biochem Soc Trans. 2010;38:1413–6.
Kunwar AJ, Rickmann M, Backofen B, Browski SM, Rosenbusch J, Schöning S, et al. Lack of the endosomal SNAREs vti1a and vti1b led to significant impairments in neuronal development. PNAS U S A. 2011;108:2575–80.
Ponten U, Ratcheson RA, Salford L, Siesjö BK. Optimal freezing conditions for cerebral metabolites in rats. J. Neurochem. 1973;21:1127–38.
Luo T, Roman P, Liu C, Sun X, Park Y, Hu B. Upregulation of the GEF-H1 Pathway after Transient Cerebral Ischemia. Experimental neurology. 2015;263:306–13.
Brunger AT. Structure of proteins involved in synaptic vesicle fusion in neurons. Annu Rev. Biophys Biomol Struct. 2001;30:157–1571.
Malhotra V, Orci L, Glick BS, Block MR, Rothman JE. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988;54:221–7.
May AP, Whiteheart SW, Weis WI. Unraveling the mechanism of the vesicle transport ATPase NSF, the N-ethylmaleimide-sensitive factor. J Biol Chem. 2001;276:21,991–4.
Whiteheart SW, Matveeva EA. Multiple binding proteins suggest diverse functions for the N-ethylmaleimide sensitive factor. J Struct Biol. 2004;146:32–43.
Brandon E, Szul T, Alvarez C, Grabski R, Benjamin R, Kawai R, et al. On and off membrane dynamics of the endoplasmic reticulum-golgi tethering factor p115 in vivo. Mol Biol Cell. 2006;17:2996–3008.
Liu CL, Hu BR. Alterations of N-Ethylmaleimide-Sensitive ATPase Following Transient Cerebral Ischemia. Neuroscience. 2004;128:767–74.
Antón-Fernández A, Aparicio-Torres G, Tapia S, DeFelipe J, Muñoz A. Morphometric alterations of Golgi apparatus in Alzheimer’s disease are related to tau hyperphosphorylation. Neurobiol Dis. 2017;97(Pt A):11–23.
Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 1824;2012:68–88.
Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–500.
Pungercar JR, Caglic D, Sajid M, Dolinar M, Vasiljeva O, Pozgan U, et al. Autocatalytic processing of procathepsin B is triggered by proenzyme activity. The FEBS journal. 2009;276:660–8.
Block MR, Glick BS, Wilcox CA, Wieland FT, Rothman JE. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci U S A. 1988;85:7852–6.
Hong HK, Chakravarti A, Takahashi JS. The gene for soluble N-ethylmaleimide sensitive factor attachment protein {alpha} is mutated in hydrocephaly with hop gait (hyh) mice. Proc Natl Acad Sci U S A. 2004;101:1748–53.
Https://gtexportal.org/home/gene/NSF
Diaz R, Mayorga LS, Weidman PJ, Rothman JE, Stahl PD. Vesicle fusion following receptor-mediated endocytosis requires a protein active in Golgi transport. Nature. 1989;339:398–400.
Wattenberg BW, Raub TJ, Hiebsch RR, Weidman PJ. The activity of Golgi transport vesicles depends on the presence of the N-ethylmaleimide-sensitive factor (NSF) and a soluble NSF attachment protein (alpha SNAP) during vesicle formation. J Cell Biol. 1992;118:1321–32.
Acharya U, Jacobs R, Peters JM, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904.
Naslavsky N, McKenzie J, Altan-Bonnet N, Sheff D, Caplan S. EHD3 regulates early-endosome-to-Golgi transport and preserves Golgi morphology. J Cell Sci. 2009;122:389–400.
Mullock BM, Bright NA, Fearon CW, Gray SR, Luzio J. Fusion of Lysosomes with Late Endosomes Produces a Hybrid Organelle of Intermediate Density and Is NSF Dependent. J Cell Biol. 1998;140(3):591–601.
Petanceska S, Burke S, Watson SJ, Devi L. Differential distribution of messenger RNAs for cathepsins B, L and S in adult rat brain: an in situ hybridization study. Neuroscience. 1994;59:729–38.
Gómez-Sintes R, Ledesma MD, Boya P. Lysosomal cell death mechanisms in aging. Ageing Res Rev. 2016;32:150–168.
Repnik U, Stoka V, Turk V, Turk B. Lysosomes and lysosomal cathepsins in cell death. Biochim Biophys Acta. 1824;2012:22–33.
Jakobson M, Jakobson M, Llano O, Palgi J, Arumäe U. Multiple mechanisms repress N-Bak mRNA translation in the healthy and apoptotic neurons. Cell Death Dis. 2013;4:e777.
Akhtar RS, Ness JM, Roth KA. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta. 1644;2004:189–203.
Serrano-Puebla A, Boya P. Lysosomal membrane permeabilization in cell death: new evidence and implications for health and disease. Ann N Y Acad Sci. 2016;1371:30–44.
Prunell GF, Mathiesen T, Svendgaard NA. Experimental subarachnoid hemorrhage: cerebral blood flow and brain metabolism during the acute phase in three different models in the rat. Neurosurgery. 2004;54:426–36.
Funding
This work was supported by National Institutes of Health (NIH) grants: NS36810, NS40407, and NS097875; by Veteran Affair Merit grant: I01BX001696; and by the American Heart Association 0940042N-5 to B.R.H.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dong Yuan, Chunli Liu, and Bingren Hu declare no conflict of interest.
Ethical Approval
This article does not contain any studies with human subjects. All the experimental procedures involving using animals were approved by the Animal Use and Care Committee in the University of Maryland School of Medicine.
Additional information
The original version of this article was revised: The author name “Dr. Jiang Wu” needed to be added as the 3rd author.
Rights and permissions
About this article
Cite this article
Yuan, D., Liu, C., Wu, J. et al. Inactivation of NSF ATPase Leads to Cathepsin B Release After Transient Cerebral Ischemia. Transl. Stroke Res. 9, 201–213 (2018). https://doi.org/10.1007/s12975-017-0571-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-017-0571-1