Abstract
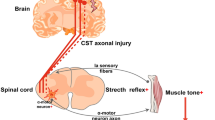
Motor impairment is the most common complication after stroke, and recovery of motor function has been shown to be dependent on the extent of lesion in the ipsilesional corticospinal tract (iCST) and activity within ipsilesional primary and secondary motor cortices. However, work from neuroimaging research has suggested a role of the contralesional hemisphere in promoting recovery after stroke potentially through the ipsilateral uncrossed CST fibers descending to ipsilateral spinal segments. These ipsilateral fibers, sometimes referred to as “latent” projections, are thought to contribute to motor recovery independent of the crossed CST. The aim of this paper is to evaluate using cumulative evidence from animal models and human patients on whether an uncrossed CST component is present in mammals and conserved through primates and humans, and whether iCST fibers have a functional role in hemiparetic/hemiplegic human conditions. This review highlights that an ipsilateral uncrossed CST exists in human during development, but the evidence on a functionally relevant iCST component in adult humans is still elusive. In addition, this review argues that whereas activity within the ipsilesional cortex is essential for enhancing motor recovery after stroke, the role of iCST projections specifically is still controversial. Finally, conclusions from current literature emphasize the importance of activity in the ipsilesional cortex and the integrity of crossed CST fibers as major determinants of motor recovery after brain injury.


Similar content being viewed by others
References
Armand J. Topical versus diffuse organization of the corticospinal tract in the cat. Journal de physiologie. 1978;74(3):227–30.
Satomi H, Takahashi K, Kosaka I, Aoki M. Reappraisal of projection levels of the corticospinal fibers in the cat, with special reference to the fibers descending through the dorsal funiculus: a WGA-HRP study. Brain Res. 1989;492(1–2):255–60.
Lacroix S, Havton LA, McKay H, Yang H, Brant A, Roberts J, et al. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J Comp Neurol. 2004;473(2):147–61. doi:10.1002/cne.20051.
Tigges J, Nakagawa S, Tigges M. Efferents of area 4 in a south American monkey (Saimiri). I. Terminations in the spinal cord. Brain Res. 1979;171(1):1–10.
Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129(Pt 3):791–808. doi:10.1093/brain/awh713.
Muller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol. 1997;42(5):705–11. doi:10.1002/ana.410420506.
Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125(Pt 10):2222–37.
Lawrence DG, Kuypers HG. Pyramidal and non-pyramidal pathways in monkeys: anatomical and functional correlation. Science. 1965;148(3672):973–5.
Herbert WJ, Powell K, Buford JA. Evidence for a role of the reticulospinal system in recovery of skilled reaching after cortical stroke: initial results from a model of ischemic cortical injury. Exp Brain Res. 2015;233(11):3231–51. doi:10.1007/s00221-015-4390-x.
Buetefisch CM. Role of the Contralesional hemisphere in post-stroke recovery of upper extremity motor function. Front Neurol. 2015;6:214. doi:10.3389/fneur.2015.00214.
Dancause N, Touvykine B, Mansoori BK. Inhibition of the contralesional hemisphere after stroke: reviewing a few of the building blocks with a focus on animal models. Prog Brain Res. 2015;218:361–87. doi:10.1016/bs.pbr.2015.01.002.
Eyre JA. Development and plasticity of the corticospinal system in man. Neural Plast. 2003;10(1–2):93–106. doi:10.1155/NP.2003.93.
Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57(9):1543–54.
Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16(20):6513–25.
Ralston DD, Ralston HJ. The terminations of corticospinal tract axons in the macaque monkey. J Comp Neurol. 1985;242(3):325–37.
Kim SH, Pohl PS, Luchies CW, Stylianou AP, Won Y. Ipsilateral deficits of targeted movements after stroke. Arch Phys Med Rehabil. 2003;84(5):719–24.
Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J Neurophysiol. 2004;92(6):3276–85. doi:10.1152/jn.00549.2004.
Marchi V, Guzzetta A, Cioni G. Cerebral plasticity and functional reorganization in children with congenital brain lesions. Neonatology. 2017:1–10.
Rehme AK, Fink GR, von Cramon DY, Grefkes C. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex. 2011;21(4):756–68. doi:10.1093/cercor/bhq140.
Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99(22):14518–23. doi:10.1073/pnas.222536799.
Bestmann S, Swayne O, Blankenburg F, Ruff CC, Teo J, Weiskopf N, et al. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci. 2010;30(36):11926–37. doi:10.1523/JNEUROSCI.5642-09.2010.
Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: a rehabilitation approach for chronic stroke. Prog Neurobiol. 2005;75(5):309–20. doi:10.1016/j.pneurobio.2005.04.001.
Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89(3):1256–64. doi:10.1152/jn.00950.2002.
Soteropoulos DS, Edgley SA, Baker SN. Lack of evidence for direct corticospinal contributions to control of the ipsilateral forelimb in monkey. J Neurosci. 2011;31(31):11208–19. doi:10.1523/JNEUROSCI.0257-11.2011.
Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28(12):2518–27.
Foltys H, Krings T, Meister IG, Sparing R, Boroojerdi B, Thron A, et al. Motor representation in patients rapidly recovering after stroke: a functional magnetic resonance imaging and transcranial magnetic stimulation study. Clin Neurophysiol. 2003;114(12):2404–15.
Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31(3):656–61.
Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129(3):809–19.
Armand J, Kuypers HG. Organization of contralateral and bilateral projections of corticospinal tracts in cats. C R Hebd Seances Acad Sci D. 1977;285(16):1455–8.
Satomi H, Takahashi K, Aoki M, Kosaka I. Anatomical evidence for the re-crossing of lateral corticospinal fibers via the posterior gray commissure in the cat spinal cord. Neurosci Lett. 1988;88(2):157–60.
Rapisarda C, Simonelli G, Monti S. Cells of origin and topographic organization of corticospinal neurons in the guinea pig by the retrograde HRP method. Brain Res. 1985;334(1):85–96.
Armand J, Kuypers HG. Cells of origin of crossed and uncrossed corticospinal fibers in the cat: a quantitative horseradish peroxidase study. Exp Brain Res. 1980;40(1):23–34.
Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386(2):293–303.
Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol. 2004;91(4):1832–9.
Nielsen JB, Perez MA, Oudega M, Enriquez-Denton M, Aimonetti JM. Evaluation of transcranial magnetic stimulation for investigating transmission in descending motor tracts in the rat. Eur J Neurosci. 2007;25(3):805–14. doi:10.1111/j.1460-9568.2007.05326.x.
Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci. 2009;29(19):6196–206. doi:10.1523/JNEUROSCI.5852-08.2009.
Hoff E, Hoff H. Spinal terminations of the projection fibers from the motor cortex of primates. Brain. 1934.
Nakagawa S. Onuf's nucleus of the sacral cord in a south American monkey (Saimiri): its location and bilateral cortical input from area 4. Brain Res. 1980;191(2):337–44.
Rosenzweig ES, Brock JH, Culbertson MD, Lu P, Moseanko R, Edgerton VR, et al. Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys. J Comp Neurol. 2009;513(2):151–63.
Yoshino-Saito K, Nishimura Y, Oishi T, Isa T. Quantitative inter-segmental and inter-laminar comparison of corticospinal projections from the forelimb area of the primary motor cortex of macaque monkeys. Neuroscience. 2010;171(4):1164–79. doi:10.1016/j.neuroscience.2010.10.007.
Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Pizzimenti MA, Darling WG. Terminal distribution of the corticospinal projection from the hand/arm region of the primary motor cortex to the cervical enlargement in rhesus monkey. J Comp Neurol. 2013;521(18):4205–35. doi:10.1002/cne.23410.
Kondo T, Yoshihara Y, Yoshino-Saito K, Sekiguchi T, Kosugi A, Miyazaki Y, et al. Histological and electrophysiological analysis of the corticospinal pathway to forelimb motoneurons in common marmosets. Neurosci Res. 2015;98:35–44. doi:10.1016/j.neures.2015.05.001.
Aizawa H, Mushiake H, Inase M, Tanji J. An output zone of the monkey primary motor cortex specialized for bilateral hand movement. Exp Brain Res. 1990;82(1):219–21.
Boudrias MH, Lee SP, Svojanovsky S, Cheney PD. Forelimb muscle representations and output properties of motor areas in the mesial wall of rhesus macaques. Cereb Cortex. 2010;20(3):704–19. doi:10.1093/cercor/bhp136.
Montgomery LR, Herbert WJ, Buford JA. Recruitment of ipsilateral and contralateral upper limb muscles following stimulation of the cortical motor areas in the monkey. Exp Brain Res. 2013;230(2):153–64. doi:10.1007/s00221-013-3639-5.
Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Hynes SM, Pizzimenti MA, et al. Vulnerability of the medial frontal corticospinal projection accompanies combined lateral frontal and parietal cortex injury in rhesus monkey. J Comp Neurol. 2015;523(4):669–97. doi:10.1002/cne.23703.
Aisaka A, Aimi Y, Yasuhara O, Tooyama I, Kimura H, Shimada M. Two modes of corticospinal reinnervation occur close to spinal targets following unilateral lesion of the motor cortex in neonatal hamsters. Neuroscience. 1999;90(1):53–67.
Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40(7):2546–51. doi:10.1161/STROKEAHA.109.547265.
Whishaw IQ, Metz GA. Absence of impairments or recovery mediated by the uncrossed pyramidal tract in the rat versus enduring deficits produced by the crossed pyramidal tract. Behav Brain Res. 2002;134(1–2):323–36.
Carmel JB, Kimura H, Berrol LJ, Martin JH. Motor cortex electrical stimulation promotes axon outgrowth to brain stem and spinal targets that control the forelimb impaired by unilateral corticospinal injury. Eur J Neurosci. 2013;37(7):1090–102.
Gonzalez CL, Gharbawie OA, Williams PT, Kleim JA, Kolb B, Whishaw IQ. Evidence for bilateral control of skilled movements: ipsilateral skilled forelimb reaching deficits and functional recovery in rats follow motor cortex and lateral frontal cortex lesions. Eur J Neurosci. 2004;20(12):3442–52. doi:10.1111/j.1460-9568.2004.03751.x.
Okabe N, Narita K, Miyamoto O. Axonal remodeling in the corticospinal tract after stroke: how does rehabilitative training modulate it? Neural Regen Res. 2017;12(2):185–92. doi:10.4103/1673-5374.200792.
Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schroter A, et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344(6189):1250–5. doi:10.1126/science.1253050.
Fouad K, Klusman I, Schwab ME. Regenerating corticospinal fibers in the marmoset (Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-a antibody IN-1. Eur J Neurosci. 2004;20(9):2479–82. doi:10.1111/j.1460-9568.2004.03716.x.
Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, et al. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12(7):790–2. doi:10.1038/nm1436.
Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135(Pt 7):2277–89. doi:10.1093/brain/aws115.
Nathan PW, Smith MC, Deacon P. The corticospinal tracts in man. Course and location of fibres at different segmental levels. Brain. 1990;113(Pt 2):303–24.
Colebatch JG, Rothwell JC, Day BL, Thompson PD, Marsden CD. Cortical outflow to proximal arm muscles in man. Brain. 1990;113(Pt 6):1843–56.
Berardelli A, Priori A, Inghilleri M, Cruccu G, Mercuri B, Manfredi M. Corticobulbar and corticospinal projections to neck muscle motoneurons in man. A functional study with magnetic and electric transcranial brain stimulation. Exp Brain Res. 1991;87(2):402–6.
Carr LJ, Harrison LM, Stephens JA. Evidence for bilateral innervation of certain homologous motoneurone pools in man. J Physiol. 1994;475(2):217–27.
Strutton PH, Beith ID, Theodorou S, Catley M, McGregor AH, Davey NJ. Corticospinal activation of internal oblique muscles has a strong ipsilateral component and can be lateralised in man. Exp Brain Res. 2004;158(4):474–9. doi:10.1007/s00221-004-1939-5.
Quartarone A, MacKinnon C, Rothwell J. Ipsilateral EMG responses in pectoralis major muscle evoked by transcranial magnetic stimulation over the motor cortex. J Physiol Paris. 1999;520:74P.
Tunstill SA, Wynn-Davies AC, Nowicky AV, McGregor AH, Davey NJ. Corticospinal facilitation studied during voluntary contraction of human abdominal muscles. Exp Physiol. 2001;86(1):131–6.
Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, et al. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol. 1999;518(Pt 3):895–906.
Ellaway PH, Davey NJ, Maskill DW, Rawlinson SR, Lewis HS, Anissimova NP. Variability in the amplitude of skeletal muscle responses to magnetic stimulation of the motor cortex in man. Electroencephalogr Clin Neurophysiol. 1998;109(2):104–13.
Tsao H, Galea MP, Hodges PW. Concurrent excitation of the opposite motor cortex during transcranial magnetic stimulation to activate the abdominal muscles. J Neurosci Methods. 2008;171(1):132–9. doi:10.1016/j.jneumeth.2008.02.005.
Kikuchi T, Matsumoto R, Mikuni N, Yokoyama Y, Matsumoto A, Ikeda A, et al. Asymmetric bilateral effect of the supplementary motor area proper in the human motor system. Clin Neurophysiol. 2012;123(2):324–34. doi:10.1016/j.clinph.2011.06.011.
Lo YL, Dan YF, Tan YE, Fook-Chong S, Tan SB, Tan CT, et al. Intraoperative monitoring study of ipsilateral motor evoked potentials in scoliosis surgery. Eur Spine J. 2006;15(Suppl 5):656–60. doi:10.1007/s00586-006-0190-0.
Fisher KM, Zaaimi B, Baker SN. Reticular formation responses to magnetic brain stimulation of primary motor cortex. J Physiol. 2012;590(16):4045–60. doi:10.1113/jphysiol.2011.226209.
Wolpaw JR, Kaas JH. Taking sides: corticospinal tract plasticity during development. Neurology. 2001;57(9):1530–1.
Brouwer B, Smits E. Corticospinal input onto motor neurons projecting to ankle muscles in individuals with cerebral palsy. Dev Med Child Neurol. 1996;38(9):787–96.
Can L, Harrison L, Evans A, Stephens J. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–47.
Farmer SF, Harrison LM, Ingram DA, Stephens JA. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology. 1991;41(9):1505.
Leonard CT, Goldberger ME. Consequences of damage to the sensorimotor cortex in neonatal and adult cats. II. Maintenance of exuberant projections. Dev Brain Res. 1987;32(1):15–30.
Nathan PW, Smith M, Deacon P. Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain. 1996;119(Pt 6):1809–33.
Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krägeloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004;56(6):854–63.
Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev. 2007;31(8):1136–49. doi:10.1016/j.neubiorev.2007.05.011.
Stanfield BB. The development of the corticospinal projection. Prog Neurobiol. 1992;38(2):169–202.
Verhaart W. Hypertrophy of pes pedunculi and pyramid al result of degeneration of contralateral corticofugal fiber tracts. J Comp Neurol. 1950;92(1):1–15.
Sebastianelli L, Versace V, Taylor A, Brigo F, Nothdurfter W, Saltuari L et al. Functional reorganization after hemispherectomy in humans and animal models: what can we learn about the brain’s resilience to extensive unilateral lesions? Brain Res Bull. 2017.
Rutten GJ, Ramsey NF, van Rijen PC, Franssen H, van Veelen CW. Interhemispheric reorganization of motor hand function to the primary motor cortex predicted with functional magnetic resonance imaging and transcranial magnetic stimulation. J Child Neurol. 2002;17(4):292–7. doi:10.1177/088307380201700411.
Pilato F, Dileone M, Capone F, Profice P, Caulo M, Battaglia D, et al. Unaffected motor cortex remodeling after hemispherectomy in an epileptic cerebral palsy patient. A TMS and fMRI study. Epilepsy Res. 2009;85(2–3):243–51. doi:10.1016/j.eplepsyres.2009.03.016.
Kupper H, Kudernatsch M, Pieper T, Groeschel S, Tournier JD, Raffelt D, et al. Predicting hand function after hemidisconnection. Brain. 2016;139(Pt 9):2456–68. doi:10.1093/brain/aww170.
Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26(22):6096–102. doi:10.1523/JNEUROSCI.4564-05.2006.
Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009;30(11):3461–74. doi:10.1002/hbm.20770.
Butefisch CM, Kleiser R, Korber B, Muller K, Wittsack HJ, Homberg V, et al. Recruitment of contralesional motor cortex in stroke patients with recovery of hand function. Neurology. 2005;64(6):1067–9. doi:10.1212/01.WNL.0000154603.48446.36.
Verleger R, Adam S, Rose M, Vollmer C, Wauschkuhn B, Kompf D. Control of hand movements after striatocapsular stroke: high-resolution temporal analysis of the function of ipsilateral activation. Clin Neurophysiol. 2003;114(8):1468–76.
Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33(2):181–9. doi:10.1002/ana.410330208.
Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101(4):316–28.
Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, et al. Corticospinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol. 2015; doi:10.1002/ana.24510.
Soderblom C, Lee DH, Dawood A, Carballosa M, Jimena Santamaria A, Benavides FD et al. 3D Imaging of Axons in Transparent Spinal Cords from Rodents and Nonhuman Primates. eNeuro. 2015;2(2). doi:10.1523/ENEURO.0001-15.2015.
Acknowledgments
W Feng, D Adkins, and S Kautz acknowledge grant support from National Institute of Health (P20GM109040 and HD086844).
W Feng acknowledges grant support from American Heart Association (14SDG1829003 and 15SFDRN26030003) and NIH/CTSA (UL1RR029882).
D Adkins acknowledges grant support from National Institute of Health (5R01NS065866-06).
S Tomlinson acknowledges grant support from National Institute of Health (P20GM109040) and the Department of Veterans Affairs (Merit Award 1I01RX001141 and 1BX001218).
A Alawieh acknowledges grant support from the American Heart Association (15PRE25250009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Alawieh, A., Tomlinson, S., Adkins, D. et al. Preclinical and Clinical Evidence on Ipsilateral Corticospinal Projections: Implication for Motor Recovery. Transl. Stroke Res. 8, 529–540 (2017). https://doi.org/10.1007/s12975-017-0551-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-017-0551-5