Abstract
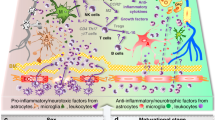
Neuroimmune processes contribute to hypoxic–ischemic damage in the immature brain and may play a role in the progression of particular variants of neonatal encephalopathy. The present study was designed to elucidate molecular mediators of interactions between astrocytes, neurons, and infiltrating peripheral immune cells after experimental neonatal hypoxia–ischemia (HI). Splenectomy was performed on postnatal day 7 Sprague–Dawley rats 3 days prior to HI surgery, in which the right common carotid artery was permanently ligated followed by 2 h of hypoxia (8 % O2). Quantitative analysis showed that natural killer (NK) and T cell expression was reduced in spleen but increased in the brain following HI. Elevations in cyclooxygenase-2 (COX-2) expression after HI by immune cells promoted interleukin-15 (IL-15) expression in astrocytes and infiltration of inflammatory cells to site of injury; additionally, these downregulated the prosurvival protein, phosphoinositide-3-kinase (PI3K), resulting in caspase 3-mediated neuronal death. The removal of the largest pool of peripheral immune cells in the body by splenectomy and COX-2 inhibitors as well as rendering NK cells inactive by CD161 knockdown significantly ameliorated cerebral infarct volume at 72 h, diminished body weight loss and brain and systemic organ atrophy, and reduced neurobehavioral deficits at 3 weeks. Herein, we demonstrate with the use of surgical approach (splenectomy) and with pharmacological loss–gain function approach using COX-2 inhibitors/agonists as well as with NK cell-type-specific siRNA that after neonatal HI, the infiltrating peripheral immune cells may modulate downstream targets of cell death and neuroinflammation by COX-2-regulated signals.






Similar content being viewed by others
References
Alvarez-Diaz A, Hilario E, de Cerio FG, Valls-i-Soler A, Alvarez-Diaz FJ. Hypoxic-ischemic injury in the immature brain—key vascular and cellular players. Neonatology. 2007;92:227–35.
Gelderblom M, Leypoldt F, Steinbach K, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–57.
Curin Y, Ritz MF, Andriantsitohaina R. Cellular mechanisms of the protective effect of polyphenols on the neurovascular unit in strokes. Cardiovasc Hematol Agents Med Chem. 2006;4:277–88.
Lo EH. Experimental models, neurovascular mechanisms and translational issues in stroke research. Br J Pharmacol. 2008;153:S396–405.
del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–82.
Zhou P, Qian L, Chou T, Iadecola C. Neuroprotection by PGE2 receptor EP1 inhibition involves the PTEN/AKT pathway. Neurobiol Dis. 2008;29:543–51.
Fathali N, Ostrowski RP, Lekic T, et al. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic–ischemic brain injury. Crit Care Med. 2010;38:572–8.
Ren K, Dubner R. Neuron–glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–9.
Gomez-Nicola D, Valle-Argos B, Pita-Thomas DW, Nieto-Sampedro M. Interleukin 15 expression in the CNS: blockade of its activity prevents glial activation after an inflammatory injury. Glia. 2008;56:494–505.
Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–70.
Ajmo CT, Vernon DO, Collier L, et al. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–34.
Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–74.
Yager JY, Wright S, Armstrong EA, Jahraus CM, Saucier DM. The influence of aging on recovery following ischemic brain damage. Behav Brain Res. 2006;173:171–80.
Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505.
Kusaka I, Kusaka G, Zhou C, et al. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 2004;286:H2442–51.
Kveberg L, Dai KZ, Westgaard IH, Fossum MS, Naper C, Vaage JT. Two major groups of rat NKR-P1 receptors can be distinguished based on chromosomal localization, phylogenetic analysis and Clr ligand binding. Eur J Immunol. 2009;39:541–51.
Vendrame M, Gemma C, Pennypacker KR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006;199:191–200.
Andine P, Thordstein M, Kjellmer I, et al. Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J Neurosci Methods. 1990;35:253–60.
Carty ML, Wixey JA, Colditz PB, Buller KM. Post-insult minocycline treatment attenuates hypoxia–ischemia-induced neuroinflammation and white matter injury in the neonatal rat: a comparison of two different dose regimens. Int J Dev Neurosci. 2008;26:477–85.
Balduini W, De Angelis V, Mazzoni E, Cimino M. Simvastatin protects against long-lasting behavioral and morphological consequences of neonatal hypoxic/ischemic brain injury. Stroke. 2001;32:2185–91.
Gee JM, Kalil A, Shea C, Becker KJ. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke. 2007;38:783–8.
Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68.
Leonardo CC, Hall AA, Collier LA, Gottschall PE, Pennypacker KR. Inhibition of gelatinase activity reduces neural injury in an ex-vivo model of hypoxia–ischemia. Neuroscience. 2009;160:755–66.
Strunk T, Hartel C, Temming P, Matzke N, Zimmer J, Schultz C. Erythropoietin inhibits cytokine production of neonatal and adult leukocytes. Acta Paediatr. 2008;97:16–20.
Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90.
Hedtjarn M, Mallard C, Hagberg H. Inflammatory gene profiling in the developing mouse brain after hypoxia–ischemia. J Cereb Blood Flow Metab. 2004;24:1333–51.
Bauer J, Rauschka H, Lassmann H. Inflammation in the nervous system: the human perspective. Glia. 2001;36:235–43.
Schroeter M, Jander S. T-cell cytokines in injury-induced neural damage and repair. Neuromolecular Med. 2005;7:183–95.
Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52:70–7.
Kakishita H, Hattori Y. Vascular smooth muscle cell activation and growth by 4-hydroxynonenal. Life Sci. 2001;69:689–97.
Kumagai T, Matsukawa N, Kaneko Y, Kusumi Y, Mitsumata M, Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 2004;279:48389–96.
Hanisch UK, Quirion R. Interleukin-2 as a neuroregulatory cytokine. Brain Res Rev. 1995;21:246–84.
Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–61.
Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403.
Diab A, Cohen AD, Alpdogan O, Perales MA. IL-15: targeting CD8+ T cells for immunotherapy. Cytotherapy. 2005;7:23–35.
Beilharz EJ, Williams CE, Dragunow M, Sirimanne ES, Gluckman PD. Mechanisms of delayed cell death following hypoxic-ischemic injury in the immature rat: evidence for apoptosis during selective neuronal loss. Brain Res Mol Brain Res. 1995;29:1–14.
Inder TE, Volpe JJ. Mechanisms of perinatal brain injury. Semin Neonatol. 2000;5:3–16.
Linker R, Gold R, Luhder F. Function of neurotrophic factors beyond the nervous system: inflammation and autoimmune demyelination. Crit Rev Immunol. 2009;29:43–68.
Davis IA, Knight KA, Rouse BT. The spleen and organized lymph nodes are not essential for the development of gut-induced mucosal immune responses in lymphotoxin-alpha deficient mice. Clin Immunol Immunopathol. 1998;89:150–9.
Novitsky YW, Litwin DE, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411–9.
Ostrowski RP, Schulte R, Ling T, Nie Y, Lee T, Manaenko A, Zhang JH. The acute splenic irradiation reduces brain injury in the rat focal ischemic stroke model. Translational Stroke Res. 2012;3(4):473–81.
Leuschner F, Panizzi P, Chico-Calero I, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–73.
Acknowledgments
The authors thank W. Tong and K. Cordero for their technical assistance on this project. This work was supported by a grant from the National Institutes of Health [NS054685 to J.H.Z.].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fathali, N., Ostrowski, R.P., Hasegawa, Y. et al. Splenic Immune Cells in Experimental Neonatal Hypoxia–Ischemia. Transl. Stroke Res. 4, 208–219 (2013). https://doi.org/10.1007/s12975-012-0239-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-012-0239-9