Abstract
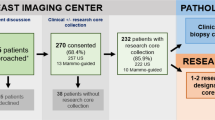
The success of personalized medicine in the oncology clinic is very dependent on the results from a translational research effort to identify individual and host molecular biomarkers to enable proper selection of anti-cancer therapy. For this to happen, it is necessary to obtain timely access to high-quality biological samples of both host and tumor tissues and biological fluids. At the Segal Cancer Center, we have initiated several prospective sample collections based on research-driven breast biopsies in different contexts, including primary and metastatic breast lesions in patients receiving specific treatments. We here describe some of the challenges involved in such a translational research program and our experience in setting up biopsy-driven research protocols, highlighting the human aspects of conducting these complex enterprises.

Similar content being viewed by others
References
Simmons C, Miller N, Geddie W, Gianfelice D, Oldfield M, Dranitsaris G, Clemons MJ (2009) Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol 20(9):1499–1504
Broom RJ, Tang PA, Simmons C, Bordeleau L, Mulligan AM, O’Malley FP, Miller N, Andrulis IL, Brenner DM, Clemons MJ (2009) Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res 29(5):1557–1562
Agulnik M, Oza AM, Pond GR, Siu LL (2006) Impact and perceptions of mandatory tumor biopsies for correlative studies in clinical trials of novel anticancer agents. J Clin Oncol 24(30):4801–4807
Peppercorn J, Shapira I, Collyar D, Deshields T, Lin N, Krop I, Grunwald H, Friedman P, Partridge AH, Schilsky RL, Bertagnolli MM (2010) Ethics of mandatory research biopsy for correlative end points within clinical trials in oncology. J Clin Oncol 28(15):2635–2640
Espina V, Mueller C, Edmiston K, Sciro M, Petricoin EF, Liotta LA (2009) Tissue is alive: new technologies are needed to address the problems of protein biomarker pre-analytical variability. Proteomics Clin Appl 3(8):874–882
De Cecco L, Musella V, Veneroni S, Cappelletti V, Bongarzone I, Callari M, Valeri B, Pierotti MA, Daidone MG (2009) Impact of biospecimens handling on biomarker research in breast cancer. BMC Cancer 24(9):409
Spruessel A, Steimann G, Jung M, Lee SA, Carr T, Fentz AK, Spangenberg J, Zornig C, Juhl HH, David KA (2004) Tissue ischemia time affects gene and protein expression patterns within minutes following surgical tumor excision. Biotechniques 36(6):1030–1037
Siddiqui S, Rimm DL (2010) Pre-analytic variables and phospho-specific antibodies: the Achilles heel of immunohistochemistry. Breast Cancer Res 12(6):113
Schlomm T, Näkel E, Lübke A, Buness A, Chun FK, Steuber T, Graefen M, Simon R, Sauter G, Poustka A, Huland H, Erbersdobler A, Sültmann H, Hellwinkel OJ (2008) Marked gene transcript level alterations occur early during radical prostatectomy. Eur Urol 53:333–344
Pinhel IF, Macneill FA, Hills MJ, Salter J, Detre S, A’hern R, Nerurkar A, Osin P, Smith IE, Dowsett M (2010) Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res 12(5):R76. [Epub ahead of print]
Bai Y, Tolles J, Cheng H, Siddiqui S, Gopinath A, Pectasides E, Camp RL, Rimm DL, Molinaro AM (2011) Quantitative assessment shows loss of antigenic epitopes as a function of pre-analytic variables. Lab Invest 91(8):1253–1261. doi:10.1038/labinvest.2011.75
Cleator SJ, Powles TJ, Dexter T, Fulford L, Mackay A, Smith IE, Valgeirsson H, Ashworth A, Dowsett M (2006) The effect of the stromal component of breast tumours on prediction of clinical outcome using gene expression microarray analysis. Breast Cancer Res 8(3):R32
Aguilar-Mahecha A, Buchanan M, Diaz Z, Aleynikova O, Kuzyk M, Borchers C, Basik M (2011) Study of pre-analytical variables in plasma and breast biopsies to be used for proteomic and genomic studies. In: 4th Annual Biospecimen Research Network (BRN) Symposium. March 2011, Bethesda, MD
Rai AJ, Vitzthum F (2006) Effects of preanalytical variables on peptide and protein measurements in human serum and plasma: implications for clinical proteomics. Expert Rev Proteomics 3(4):409–426
Aguilar-Mahecha A, Kuzyk M, Domansky D, Borchers C, Basik M Impact of pre-analytical variability due to blood sample collection and processing on the measurement of circulating cytokines and mid to high abundant protein biomarkers. (In preparation)
Rai AJ, Gelfand CA, Haywood BC, Warunek DJ, Yi J, Schuchard MD, Mehigh RJ, Cockrill SL, Scott GB, Tammen H, Schulz-Knappe P, Speicher DW, Vitzthum F, Haab BB, Siest G, Chan DW (2005) HUPO Plasma Proteome Project specimen collection and handling: towards the standardization of parameters for plasma proteome samples. Proteomics 5(13):3262–3277
Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S, Wang W, Brenner DE (2009) Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res 8(1):113–117
Acknowledgments
This work was supported by funding from the Fonds de la Recherche en Santé du Québec Réseau de Cancer and from Genome Québec.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguilar-Mahecha, A., Diaz, Z., Buchanan, M. et al. Making personalized medicine a reality: the challenges of a modern translational research biopsy-driven program in an academic setting: the Segal Cancer Center experience. J Med Pers 9, 104–111 (2011). https://doi.org/10.1007/s12682-011-0100-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12682-011-0100-z