Abstract
Non-small cell lung cancer (NSCLC) is the malignant tumor with the highest morbidity and leading cause of death worldwide, whereas its pathogenesis has not been fully elucidated. Although mutations in some crucial genes in WNT pathways such as β-catenin and APC are not common in NSCLC, the abnormal signal transduction of WNT pathways is still closely related to the occurrence and progression of NSCLC. WNT ligands (WNTs) are a class of secreted glycoproteins that activate WNT pathways through binding to their receptors and play important regulatory roles in embryonic development, cell differentiation, and tissue regeneration. Therefore, the abnormal expression or dysfunction of WNTs undoubtedly affects WNT pathways and thus participates in the pathogenesis of diseases. There are 19 members of human WNTs, WNT1, WNT2, WNT2b, WNT3, WNT3a, WNT4, WNT5a, WNT5b, WNT6, WNT7a, WNT7b, WNT8a, WNT8b, WNT9a, WNT9b, WNT10a, WNT10b, WNT11 and WNT16. The expression levels of WNTs, binding receptors, and activated WNT pathways are diverse in different tissue types, which endows the complexity of WNT pathways and multifarious biological effects. Although abundant studies have reported the role of WNTs in the pathogenesis of NSCLC, it still needs further study as therapeutic targets for lung cancer. This review will systematically summarize current research on human WNTs in NSCLC, from molecular pathogenesis to potential clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The latest statistics on global cancer data show that lung cancer has become the most common type of cancer with the highest morbidity and leading cause of death worldwide and also in China, among which the incidence and mortality of men rank first, and those of women rank third and second, respectively [1]. According to the histopathological characteristics, lung cancer is divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC accounts for about 85% of the total incidence of lung cancer and mainly includes three types: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma [2]. Lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) account for 60–70% of all lung cancers. Although surgery is regarded as the first choice of treatment for lung cancer, about 70% of patients have progressed to metastasis at diagnosis, or relapse after initial surgery or radiotherapy [3]. At present, the 5-year survival rate of NSCLC patients is as low as 15%, the time from diagnosis to death for most patients in the advanced stage is less than 18 months, and the therapeutic effects of surgery, radiotherapy, and chemotherapy are poor [4, 5].
Over the past decade, immunotherapy and targeted therapy have made substantial progress and significantly prolonged the progression-free survival (PFS) of patients with NSCLC [6, 7]. However, immune checkpoint inhibitors (ICIs) and targeted therapies also bring about some immune-related adverse events [12,13,14]and other unexpected adverse reactions such as thrombocytopenia, hypertension, and hyponatremia [8,9,10]. Therefore, it is still necessary to strengthen the research on these drugs to prevent these adverse reactions and to elucidate some other underlying molecular pathogenesis of NSCLC. The pathogenesis of NSCLC is very intricate, involving the abnormal transduction of many signaling pathways, including WNT, tyrosine kinase, Notch, EGFR, Hedgehog, etc. EGFR, which promotes malignant proliferation, metastasis, and angiogenesis of cancer cells by activating intracellular RAS/RAF/MEK/MAPK, PI3K/PTEN/AKT and STAT3 signaling pathways, and inhibits apoptosis of cancer cells, is expressed in over 60% of lung cancer and regarded as an important target for prognosis evaluation and treatment of NSCLC [11]. Abnormal activation of the Hedgehog pathway also enhances the stemness of cancer stem cells (CSCs) and the proliferation of cancer-associated fibroblasts (CAFs) [12]. In addition, the occurrence, metastasis and radiotherapy tolerance of NSCLC are also related to the over-activation of Notch pathway, and abnormal increase in the expression of Notch1 and Notch3 is detected in about 30–40% of NSCLC cases [13, 14]. As conserve pathways that determine the embryonic development and tissue homeostasis in multicellular organisms, WNT signaling pathways play important role in regulating the expression of genes involved in multiple cellular processes, including cell differentiation, proliferation, migration and apoptosis. Therefore, its dysregulation undoubtedly results in disease etiology like tumorigenesis of NSCLC [15, 16].
Although several elaborated review articles have systematically discussed the role of WNT signaling pathways in lung physiology and their dysregulation in the process of lung pathology such as NSCLC lesions, little is known regarding the role of different WNT ligands (WNTs) in the occurrence and progression of NSCLC [17,18,19,20]. Here, we will summarize the current insights into the WNTs in NSCLC, from molecular pathogenesis to clinical practice.
2 The mechanisms of signal transduction of WNT pathways
2.1 WNT ligands
WNT family contains many homologous genes that are highly conserved during evolution. The WNT gene is named after the wingless (wg) of Drosophila and the int-1 gene of mice [21]. Mutations in the wg gene in Drosophila could produce morphological defects, and the insertion of the mammary tumor virus gene also activates the int-1 gene in mice and promotes tumor formation [22]. Subsequently, a variety of WNT homologous genes were found in most organisms, from nematodes to humans. Among them, Drosophila and mice have 4 and at least 18 WNT genes, respectively [23]. At present, 19 WNT genes have been found in humans, which encode WNT1, WNT2, WNT2b, WNT3, WNT3a, WNT4, WNT5a, WNT5b, WNT6, WNT7a, WNT7b, WNT8a, WNT8b, WNT9a, WNT9b, WNT10a, WNT10b, WNT11 and WNT16 proteins [24]. These cysteine-rich secretory proteins act on different cells and perform a series of functions through paracrine or autocrine [25, 26]. It is widely believed that the aberrant expression of WNT ligands and mediated dysregulation of different WNT signaling pathways exert very important role in the occurrence and progression of most human malignancies, including cancers of the nervous system, the digestive system, the respiratory system, the urogenital system and the musculoskeletal system [17, 27,28,29,30,31,32].
2.2 The signal transduction of the canonical WNT pathway
According to different downstream signaling cascades, WNT signaling pathways are divided into β-catenin-dependent canonical WNT pathway and β-catenin-independent noncanonical WNT/Ca2+ and WNT/planar cell polarity (PCP) pathways, [33, 34] among which the mechanism in the signal transduction of canonical WNT pathway is much clearer. Specifically, in the absence of WNTs, β-catenin is phosphorylated by the destruction complex composed of GSK3β, CK1α, AXIN and APC in the cytoplasm, and then degraded by E3 ligase SCFβTrCP-mediated ubiquitination. CK1α phosphorylates β-catenin at serine 45 (S45) and GSK3β phosphorylates β-catenin at S33, S37 and threonine 41. AXIN and APC are responsible for the coupling of β-catenin to GSK3β. Therefore, the truncated mutation of APC could disrupt its binding ability to AXIN and affect the recruitment of β-catenin by the destruction complex. When the secreted WNTs are sufficient, they will bind to different frizzled (FZD) receptors and co-receptors LRP5/6, phosphorylate the intracellular proline-serine-rich regions in LRP6, and then recruit and inhibit GSK3β. DVL is also recruited to bind to the intracellular domain of FZD via its DEP domain to form oligomers, followed by AXIN and GSK3β, where AXIN will bind to the DIX domain of DVL. Therefore, the dissociation of AXIN from the destruction complex and binding to DVL determines the switch of the canonical WNT pathway from an inactive state to an active state. Elevated AXIN levels inactivate the WNT pathway, while elevated DVL levels exert an opposite effect. Because of the dissociation of the destruction complex, β-catenin is accumulated in the cytoplasm and translocated into the nucleus to bind to DNA-binding proteins such as TCF/LEF transcription factors, relieve the inhibitory effect of Groucho transcription inhibitors on TCF/LEF, and recruit transcription co-activators CBP and p300 to activate TCF/LEF, thus regulating the transcription of downstream target genes that determine cell proliferation, differentiation and apoptosis [35, 36]. Therefore, the abnormal signal transduction of the canonical WNT pathway will activate the transcription of a variety of tumor-related target genes, such as c-Myc, Cyclin D1, and vascular endothelial growth factor (VEGF), thereby inducing the malignant transformation of normal cells or promoting the malignant proliferation and metastasis of cancer cells and tumor angiogenesis [37,38,39]. In addition, the canonical WNT pathway also interacts with TGF-β and Notch pathways to promote tumorigenesis and epithelial-mesenchymal transition (EMT) processes [40]. Interestingly, activation of certain noncanonical WNT pathway, such as WNT5a/Ca2+ pathway, can even inhibit the canonical WNT pathway [41]. In conclusion, canonical WNT pathway is an important pathway that determines the occurrence, progression, and metastasis of cancers. Therefore, it is of great significance to further clarify its molecular mechanism for the diagnosis and treatment of malignancies [35, 42].
3 The role and action mechanisms of WNTs in tumorigenesis and progression of NSCLC
The expression levels, the binding receptors, and activated WNT pathways are different due to the large number of WNT family members. However, there is growing evidence that the aberrant expression of WNTs is closely related to the occurrence and progression of NSCLC, which may serve as important indicators for the early diagnosis and prognosis evaluation of NSCLC [26].In the following sections, we specifically focus on the changes and action mechanisms of different WNTs (Table 1) in the pathogenesis of NSCLC (Figs. 1 and 2), thus providing novel biomarkers and drug targets for the diagnosis and treatment of NSCLC.
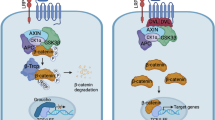
Regulatory functions of oncogenic WNTs and related upstream regulators on the canonical WNT pathway in NSCLC. In the absence of WNTs, the cytoplasmic β-catenin is phosphorylated by CK1α and GSK3β in destruction complex, followed by ubiquitination by its cognate E3 ligase, SCFβTrCP. When the secreted WNTs are sufficient, they will bind to different FZD receptors (FZDs) and co-receptors LRP5/6, phosphorylate LRP6 and then recruit DVL to the intracellular domain of FZD to form oligomers, then, AXIN and GSK3β dissociate from the destruction complex and bind to DVL. Therefore, β-catenin can be accumulated in the cytoplasm and then translocated into the nucleus to bind to DNA-binding proteins such as TCF/LEF transcription factors, and recruit transcription co-activators CBP and p300 to activate TCF/LEF, thus regulating the transcription of downstream tumor-related target genes that determine cellular proliferation, differentiation and apoptosis, such as c-Myc, cyclinD1, MMPs and survivin. During the onset and progression of NSCLC, Let-7c, miR-383, miR-148a, lncRNA-MIR-503HG, miR-185-5p, miR-326, miR-577, miR-107, miR-326, miR-1278, miR-876-5p, miR-885-3p, cicFOXP1, GPC5, GPRC5A and TRIM8 could block the signaling transduction of the canonical WNT pathway by inhibiting the activities of WNTs or down-regulating their expression, whereas circFOXP1, lncRNA RPPH1, GOLPH3, smoke, PM2.5, lncRNA-PCAT6, circ101675, circVAPA and circTUBGCP3 could activate the canonical WNT pathway by over-activating WNTs or up-regulating their expression. APC adenomatosis polyposis coli, β-TRCP β-transducin repeat-containing protein, CBP CREB-binding protein, CK1α casein kinase 1α, DVL disheveled, FOXP1, forkhead box protein P1, FZDs frizzleds, GDK-100017 2,3,6-trisubstituted quinoxaline derivative, GOLPH3 Golgi phosphoprotein 3, GPC5 glypican-5, GPRC5A G protein coupled receptor family C group 5 type A, GSK3β glycogen synthase kinase 3β, LEF lymphoid enhancer-binding factor, MMPs matrix metalloproteinases, NSCLC non-small cell lung cancer, PCAT6 prostate cancer-associated transcript 6, RPPH1 ribonuclease P RNA component H1, TCF T-cell factor, TRIM8 tripartite motif-containing 8, TUBGCP3 gamma tubulin complex component 3
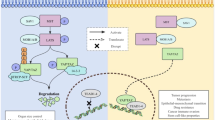
Regulatory functions of anti-cancer WNTs by activating the noncanonical WNT pathways in NSCLC. A WNT5a and WNT11 bind to FZDs and G protein subunit to recruit DVL, which triggers PLC activity and promotes intracellular Ca2+ release, and further activates calcium-dependent PKC and CAMKII signaling pathways, thus to regulate the expression of downstream genes to promote the proliferation and invasion of NSCLC cells by inducing the accumulation of nuclear NFAT transcription factors. WNT5a also inhibits VEGF-A-induced endothelial cell migration and motility to promote angiogenesis by inducing miR-27b and direct consequence of PPARγ reduction in NSCLC cells. Smoke and ATF-2 could activate the noncanonical WNT pathway by up-regulating the expression of WNT5a and WNT11. B The β-catenin-independent Wnt/PCP/JNK pathway is initiated by the cumulative binding of WNT7a to the ROR1/2-FZDs complex, then DVL is activated to bind to some small Rho GTPases such as RAC1 and RhoA to trigger JNK. This results in the inhibition of transformed cell growth but enhancement of migration and invasion of NSCLC cells. The binding of WNT7a to FZD9 inhibits cellular transformation and proliferation of NSCLC cells by activating the tumor suppressor PPARγ via the ERK5-dependent pathway, thus to relieve the inhibitory effect of MDM2 on p53 tumor suppressor pathway by inducing the expression of anti-cancer miR-29b, and promotes epithelial differentiation through activating JNK pathway and the resultant upregulation of cadherins. Wnt7a also triggers the cellular senescence of lung cancer via the inactivation of Skp2, a key negative regulator of cellular senescence. CAMKII calmodulin-dependent protein kinase II, DVL disheveled, ERK5 extracellular signal-regulated kinase 5, JNK JUN N-terminal kinase, MDM2 murine double minute 2, NFAT nuclear factor of activated T cells, NSCLC non-small cell lung cancer, PCP planar cell polarity, PKC protein kinase C, PLC phospholipase C, PPARγ peroxisome proliferator–activated receptor-γ, RAC1 Rac family small GTPase 1, ROR1/2 receptor tyrosine kinase-like orphan receptor 1/2, Skp2 S-phase kinase-associated protein-2, VEGF-A vascular endothelial growth factor-A
3.1 WNT1
WNT1 belongs to the proto-oncogene family and is highly conserved in evolution. Its expression level was increased in lung cancer, prostate cancer, and cervical cancer, [43, 44] elevated WNT1 expression was also detected in over 1/3 of NSCLC cases, and was positively correlated with the expression levels of β-catenin, Cyclin D1, and c-Myc. In addition, regardless of the TNM stage of NSCLC, the increased expression levels of WNT1 and β-catenin foreshadowed poor prognosis of NSCLC patients after surgery, [45] suggesting that the increased expression level of WNT1 is an important mechanism leading to the over-activation of canonical WNT pathway in NSCLC. WNT1 not only accelerates the proliferation of tumors by upregulating c-Myc, but also promotes the tumor malignant proliferation and angiogenesis by inducing the expression of target genes such as Cyclin D1, VEGF-A, and matrix metalloproteinase 7 (MMP7) [45, 46]. Survivin is an important inhibitor of apoptosis protein and a target gene of the canonical WNT pathway. Interestingly, its expression level was significantly positively correlated with WNT1 in NSCLC tissues, and its elevated expression level was also an important indicator of poor prognosis [47]. Therefore, WNT1 could be used as a potential drug target to treat NSCLC. Previous studies have reported that some noncoding RNAs, such as miR-383, miR-148a and MIR503HG, could inhibit the migration and invasion of NSCLC cells by targeting the expression of WNT1, induce apoptosis and reduce the tumorigenicity of cancer cells in vivo [48,49,50]. On the contrary, circFOXP1 could relieve the inhibitory effect of miR-185-5p on WNT1 and promote the progression of LUAD [51]. Recently, some studies even found that WNT1 contributes to the pathogenesis of lung cancer by regulating the tumor immune microenvironment. The expression level of WNT1 was inversely correlated with T cell abundance in LUAD tissues. LUAD cells could inhibit the expression of CC/CXC chemokine in intratumoral conventional dendritic cells (cDCs) by using paracrine WNT1 signaling and induce T cell cytotoxicity and immune resistance. Therefore, silencing WNT1 may be a valuable immunotherapeutic strategy to prevent the progression of LUAD [52]. In addition, elevated plasmic WNT1 protein was correlated with the poor prognosis of advanced NSCLC patients during the treatment of ICIs, which can be attributed to the activation of the canonical WNT pathway mediated by WNT1 [53]. WNT1 is also a target of Let-7c and inhibited by it through increased methylation, high Let-7c could therefore suppress EMT and further potentiate the osimertinib action on NSCLC cells with EGFR T790M mutations [54]. In conclusion, WNT1 promotes the pathogenesis of lung cancer, and inhibition of its overexpression by appropriate methods has a potential therapeutic effect on this disease.
3.2 WNT2
As an important member of the WNT family, WNT2 mainly functions as an oncogene [55]. Over-activation of the canonical WNT pathway mediated by WNT2 was found in many types of cancers, including the colorectal cancer, gastric cancer, breast cancer, and lung cancer [56,57,58]. Studies have found that the expression level of WNT2 protein in NSCLC tissues and serum of LUAD patients was significantly increased, and was correlated with poor outcomes of patients, it is therefore an important indicator for the diagnosis and prognosis evaluation of NSCLC [55, 59]. In NSCLC cell lines, silencing WNT2 by siRNA or antagonizing WNT2 by anti-WNT2 monoclonal antibody could reduce the cytoplasmic β-catenin level and TCF/LEF transcriptional activity, and induce cellular apoptosis. Similarly, overexpression of construct dominant negative WNT2 reduced the tumorigenicity of NSCLC cells in vitro and in vivo via inhibiting FZD8-mediated activation of WNT2/β-catenin pathway [60]. Moreover, a small molecule inhibitor 2,3,6-trisubstituted quinoxaline derivative, GDK-100,017, was found to inhibit cell proliferation of WNT2 overexpressing NSCLC cells and enhance their sensitivity to radiotherapy in a dose-dependent manner by targeting the canonical WNT pathway [61]. Therefore, targeted silencing of WNT2 expression may be a new therapeutic approach to treat NSCLC.
3.3 WNT2b
As a paralogue of WNT2, WNT2b also promotes the progression and metastasis of malignancies such as head and neck squamous cell carcinoma, malignant pleural mesothelioma, ovarian cancer, and pancreatic cancer, enhances chemotherapy resistance, and leads to poor prognosis by activating the canonical WNT pathway [62,63,64,65,66,67,68]. Both mRNA and protein expression levels of WNT2b were elevated in NSCLC cell lines, and overexpression of WNT2b promoted the proliferation, colony formation, and EMT process of NSCLC cells [64]. Interestingly, WNT2b and WNT5a are highly expressed in NSCLC cells and stromal cells and may induce the polarization of tumor-associated macrophages (TAMs) to M2 status to strengthen the tumor progression [69]. On the contrary, miR-577 inhibited the proliferation and EMT of NSCLC cells by interfering with the expression of WNT2b and related canonical WNT pathway, while lncRNA RPPH1 relieved the inhibitory effect of miR-326 on WNT2b expression, and enhanced the invasion ability, EMT and CDDP resistance of NSCLC cells [64, 70]. Therefore, inhibition of WNT2b expression has a potential therapeutic effect on NSCLC, for example, the adenoviral vector carrying shRNA against WNT2b not only induced the apoptosis of several Wnt2b-overexpressing human tumor cells by downregulating c-Myc and survivin, but also exerted a strong antitumor activity in the intrapleural lung cancer model of Wnt2b-overexpressing lung cancer xenografts [71, 72].
3.4 WNT3
As an important member of the WNT family, WNT3 promotes the occurrence and progression of many malignancies, including liver cancer, gastric cancer, and colorectal cancer, by activating the canonical WNT pathway [73,74,75,76]. Compared with that in normal tissues, the expression of WNT3 in lung cancer tissues was significantly increased and positively correlated with the expression levels of c-Myc, survivin, and Ki-67. In addition, the expression level of WNT3 in LUSC was higher than that in LUAD, and a higher WNT3 level predicted stronger invasiveness of NSCLC [77]. On the contrary, knockdown of WNT3 in NSCLC cells suppressed cellular proliferation, invasion and metastasis, and induced apoptosis by inhibiting the canonical WNT pathway [78]. Therefore, WNT3 may be another target to treat NSCLC.
3.5 WNT3a
WNT3a is highly homologous to WNT3, but with a 15% difference in the amino acid sequences. WNT3a is highly expressed in gastric cancer, colorectal cancer, prostate cancer, and breast cancer, and could enhances the development and metastasis of these malignancies by activating ERK and canonical WNT pathways [75, 79]. Moreover, WNT3a was found to increase the metastatic abilities of NSCLC cells by promoting the expression of Notch3, N-cadherin and vimentin, and cause EMT morphological changes and F-actin reorganization [80]. Recently, Song et al. found Golgi phosphoprotein 3 (GOLPH3) was overexpressed in NSCLC tissues, and promoted the secretion of exosomal WNT3a and activation of canonical WNT pathway via increasing exosome-localized cytoskeleton-associated protein 4, thus enhancing the metastasis and CSC-like phenotype in NSCLC [81]. Interestingly, fine particulate matter (PM2.5) also increases the risk of lung cancer by promoting WNT3a levels in secreted exosomes and subsequent activation of the canonical WNT pathway. However, these exosomes only enhanced the proliferation of NSCLC cells and exhibited no effect on their invasion and migration [82]. Glypican-5 (GPC5) is a member of heparin sulfate proteoglycan and exerts as a metastasis suppressor in LUAD. Wang et al. found that the expression levels of GPC5 and WNT3a were negatively correlated in LUAD, and GPC5 could suppress the proliferation and metastasis of LUAD cells by inactivating the canonical WNT pathway by competitively binding to WNT3a [83]. In addition, miR-107 is another negative regulator of WNT3a, which inhibits the invasion and EMT of NSCLC cells by suppressing WNT3a and FGF7 expression [84]. In conclusion, these findings provide a theoretical basis for using WNT3a as a drug target to treat lung cancer.
3.6 WNT5a
WNT5a is a para- and autocrine β-catenin-independent ligand that has been shown to inhibit or induce cancer [85, 86]. The expression level of WNT5a is elevated in some cancers and exerts as an oncogene, such as breast cancer, pancreatic cancer, prostate cancer, and gastric cancer [87,88,89]. In contrast, WNT5a inhibits the progression of breast cancer and liver cancer [90, 91]. Therefore, its expression level and function depend on specific cancer types and tumor microenvironments. The expression level of WNT5a was increased in over 60% of NSCLC cases, especially in lung tumors obtained from smokers and male patients. Moreover, its expression level was higher in LUSC tissues than that in LUAD counterparts [92]. Previous studies have shown that cigarette smoke induced lung carcinogenesis by activating the noncanonical WNT5a/PKC signaling and AKT, or by relieving the inhibitory effect of miR-487b on WNT5a expression and mediated activation of canonical WNT pathway [93, 94]. The expression of WNT5a was positively correlated with the expression of β-catenin, VE-cadherin, MMP2, MMP9 and VEGF-A in NSCLC [92, 95]. Overexpression of WNT5a could promote the colony formation, migration, invasion, EMT and metastasis by activating the canonical WNT pathway [92, 96]. In addition, WNT5a in NSCLC cells could increase the expression of β-catenin and VEGF-A in stromal cells through tumor-stroma interaction, thus promoting tumor angiogenesis [95]. Consistently, high level of WNT5a also inhibits VEGF-A induced angiogenesis in NSCLC squamous cells by inducing miR-27b and the direct consequence of PPARγ reduction [97]. Therefore, NSCLC patients with higher WNT5a expression levels had advanced TNM stages and poor outcomes [95, 98]. On the contrary, silencing WNT5a expression inhibited the malignant phenotype of NSCLC [96, 99]. Recently, miR-1278 and miR-876-5p were found to inhibit the progression of NSCLC by directly downregulating the expression of WNT5a, whereas circ0101675 and circVAPA acted as competing endogenous RNA to relieve the inhibition of miR-1278 and miR-876-5p on WNT5a separately [100, 101]. Similarly, miR-665 activated the WNT5a/β-catenin pathway by inhibiting the expression of TRIM8, enhancing the malignant progression of LUSC [102]. In contrast, sevoflurane, a volatile anesthetic frequently used in surgery, could inhibit the proliferation and invasion, and induce cancer cell apoptosis of LUAD and SCLC cells by blocking the lncRNA PCAT6/miR-326/WNT5a/β-catenin pathway [103]. GPRC5A is a lung tumor suppressor and is often expressed at a low level in smoking lung cancer patients. A recent study has found that cigarette smoke extract could inhibit the expression of GPRC5A in normal human lung epithelial cells and lung cancer cells, inducing the expression of WNT5a and the pathogenesis of lung cancer [104]. However, WNT5a is not always functioning through the activation of the canonical WNT pathway. Zhang et al. found that ATF-2 enhanced the proliferation and invasion of NSCLC cells by activating WNT5a/Ca2+ pathway [105]. In addition, the activation of WNT5a/PKC pathway could enhance the stemness of NSCLC cells and inhibit cell apoptosis and their sensitivity to chemotherapies by increasing endoplasmic reticulum release of Ca2+, PKC, and CaMKII and the subsequent activation of NF-kB signaling. This effect was strikingly reversed by PKC inhibitor GF109203X, [106] which also provides new insights for further understanding the role of noncanonical WNT pathways in lung carcinogenesis and chemoresistance. Reference: Kindly check whether the inserted [Page range] for references [31, 53, 69, 81, 86, 96, 124, 127, 148, 166] are appropriate.Patients with advanced NSCLC are prone to have brain metastasis and poor prognosis, and may be enhanced by EGFR mutation. However, a recent study reported that WNT5a protein was significantly decreased in brain metastasis samples and EGFR-mutant tissues because of the activation of its upstream negative regulatory ERK1/2-E2F1 pathway. Overexpression of WNT5a could inhibit the progression of EGFR mutant NSCLC by blocking the canonical WNT pathway, [107] indicating that WNT5a plays an anti-cancer role in the development of such NSCLC. Therefore, the role of WNT5 in the pathogenesis of NSCLC needs to be studied further.
3.7 WNT5b
WNT5b is highly homologous to WNT5a, but 18% difference in the amino acid sequences. WNT5b could activate the canonical and noncanonical WNT pathways, and may play an opposite role in different types of cancers [108]. Elevated expression of WNT5b promoted the progression of oral squamous cell carcinoma and breast cancer but predicted a better prognosis for glioma patients [108,109,110]. WNT5b is also highly expressed in LUAD and is positively associated with the TNM stage and poor prognosis. Therefore, overexpressing its negative upstream regulator, miR-5587-3p could suppress the progression of LUAD by interfering cell cycle and modulating amino acid metabolism [111]. Interestingly, WNT5b-associated exosomes secreted from colorectal cancer cells and pancreatic cancer cells could stimulate the migration and proliferation of NSCLC cells in a paracrine manner [112]. However, there is a still lack of study on the effect and action mechanism of WNT5b in the pathogenesis of NSCLC, and needs further study.
3.8 WNT7a
Unlike most WNTs, WNT7a can activate the canonical and noncanonical WNT pathways, but only exerts an anti-cancer effect on lung cancer. The expression of WNT7a is frequently decreased in NSCLC, accompanied by the downregulation of β-catenin and E-cadherin levels [113]. WNT7a not only induces cellular senescence by inactivating S phase kinase-associated protein 2 in a β-catenin-independent manner, [114] but also inhibits the progression of lung cancer by activating E-cadherin expression in a β-catenin-dependent manner [113]. On the contrary, WNT7a-null mice displayed E-cadherin to N-cadherin switch and a decrease in the expression of cell senescence markers and related phenotype, suggesting the increased lung tumorigenesis of these mice [114]. Strikingly, several studies performed by Winn and colleagues have found that direct binding of WNT7a to WNT receptor FZD9 in NSCLC cells could inhibit cellular transformation and proliferation by increasing the activity of a tumor suppressor PPARγ in a Gα16/ERK5 pathway-dependent manner and resultant disinhibitory effect of MDM2 on p53 tumor suppressor pathway by inducing the expression of anti-cancer miR-29b, and promote epithelial differentiation of these cells through activating JNK pathway and the resultant upregulation of cadherins [115,116,117,118]. Interestingly, WNT7a gene is located at the chromosome 3p25 region, which is known as a predilection site of homozygous deletion of many anti-cancer genes [119]. In addition, there is a high percentage of methylation in the promoter region of WNT7a gene in NSCLC tissues, which is positively correlated with the advanced TNM stage and may be related to the increased expression of DNA methyltransferase 1 induced by cigarette smoke condensate [120, 121]. However, a recent study reported that WNT7a exerted an antiproliferative effect on NSCLC cells by activating the noncanonical JNK pathway, but enhanced their migration and invasion abilities [122]. In conclusion, WNT7a is an important molecule that inhibits the occurrence and progression of NSCLC, and restoring its expression could be a valuable therapeutic strategy for treating NSCLC.
3.9 WNT11
WNT11 is most homologous to WNT4, but still with 59% difference in the amino acid sequences. Most studies have shown that WNT11 promoted the development of multiple malignancies, such as breast cancer, colon cancer, and prostate cancer, by activating the canonical and noncanonical WNT pathways [123,124,125]. However, WNT11 was found to inhibit the proliferation and metastasis of liver cancer cells via activating the noncanonical PKC pathway but suppressing the canonical WNT pathway [126]. Although WNT11 is upregulated in LUSC tissues, it inhibits cellular adhesion of NSCLC cells via suppressing the expression of E-cadherin and canonical WNT pathway, but activating nonclassical WNT pathway [127]. WNT11 is also lowly expressed in chemo-resistant SCLC cells, indicating its upregulation may contribute to the treatment of lung cancer [128]. Recently, Ito and colleagues found that the increased acetylation level of H3K27, an enhancer of the WNT11 gene caused by the upregulation of ASCL1 and its recruitment to oncogenic SOX2 may be an initiating factor in the cause of WNT11 upregulation and resultant progression of lung cancer [129, 130]. In addition, ATF-2 is found to enhance the proliferation and invasion of NSCLC cells by inducing the expression of WNT11 and mediated activation of WNT11/Ca2+ pathway [105]. In short, WNT11 is mainly carcinogenic in the development of lung cancer, and inhibiting its overexpression may aid the treatment of this disease.
3.10 The other WNTs
The latest bioinformatics analysis on NSCLC datasets showed that the expressions of WNT4, WNT9a, and WNT9b were decreased in LUAD tissues, whereas expressions of WNT6, WNT7b, WNT10a, WNT10b and WNT16 were increased in LUAD and LUSC tissues [131]. GATA4 is an important tumor suppressor in lung cancer and could induce the senescence of lung cancer cells via upregulating several miRNAs that target TGFB2 mRNA and ensuing downregulation of WNT7b expression, suggesting WNT7b may play a carcinogenic role in lung cancer [132]. WNT10b exerts a similar role in this pathogenesis because circTUBGCP3 promotes the progression of LUAD by competitively binding miR-885-3p to relieve its inhibition on WNT10b/β-catenin pathway [133]. Unfortunately, the role and action mechanism of WNT4, WNT6, WNT8a, WNT8b, WNT9a, WNT9b, WNT10a, and WNT16 in the pathogenesis of NSCLC has not been reported and needs more studies.
4 The application of WNT-based drugs in the treatment of NSCLC
4.1 Overall profile
In recent years, cancer therapies targeting WNT pathways have attracted more attention with the deeper understanding of the signal transduction mechanisms of WNT pathways, and the development of multiple targeted drugs is ongoing. The pharmacological mechanisms of these drugs specifically include the following aspects: inhibiting the secretion of WNTs and their binding to receptors; reducing the expression of β-catenin or inactivating its activity; blocking the signaling transduction of canonical WNT pathway, restoring the function or expression of certain negative regulators in this pathway, and improving the sensitivity of resistant cancer cells to anti-NSCLS drugs (Table 2).
4.2 Inhibiting the activity of WNTs
The normal secretion of WNTs and binding to receptors depend on their lipidation with palmitic acid at two conserved serine residues by an acyltransferase named porcupine (PORCN) at the post-translational level, and most WNTs are upregulated in lung cancer tissues. Therefore, inhibiting the expression or activity of Porcupine could theoretically reduce the over-activation of WNT pathways. It has been reported that silencing Porcupine expression reduced the expression of β-catenin in NSCLC cells [24]. However, Porcupine is indispensable for the maintenance of the physiological WNT pathways, excessive inhibition in it may have potentially toxic effects on normal tissues. As a specific inhibitor of Porcupine, LGK974 significantly inhibited the progression of NSCLC in vitro and in vivo, and prolonged the survival time of mice with advanced LUAD. Strikingly, the formation of cyclodextrin: LGK974 inclusion complexes could enhance the solubility and bioavailability of LGK974 in mice and reduce its intestinal toxicity [134]. Unfortunately, none of Porcupine-related inhibitor is commercially available, and LGK974 (NCT01351103), ETC-159 (NCT02521844), CGX1321 (NCT02675946), and RXC004 (NCT03447470) are still tested in phase I clinical trials [135].
4.3 Competitive binding to WNT receptors
The competitive binding of WNT receptors is another effective strategy to inhibit the canonical WNT pathway. OMP-18R5 is a monoclonal antibody targeting FZD7, which can competitively bind to five FZDs due to the high homology of FZD family members. Therefore, this antibody could inhibit the growth of NSCLC cells in vitro and in vivo by blocking the activation of the canonical WNT pathway induced by WNTs [136]. Similarly, the fusion protein OMP-54F28 could bind to the cysteine-rich domain of FZD8 with its Fc domain to antagonize WNT signaling, suppressing the growth of multiple cancers and tumorigenicity of CSCs [137].
4.4 Promoting the degradation of β-catenin
Currently, a variety of small molecule inhibitors and natural compounds targeting β-catenin have been developed, given its essential role in the canonical WNT pathway. Tankyrases are important regulators in the canonical WNT pathway, XAV939 specifically binds to them to stabilize AXIN in the destruction complex, thus promoting the phosphorylation and subsequent degradation of β-catenin. Interestingly, XAV939 has been reported to inhibit the proliferation and migration of NSCLC cells by targeting the canonical WNT pathway. Moreover, the combination use of XAV939 and cisplatin improved the therapeutic effect of cisplatin and reduced its adverse reactions [138]. The activating mutation of EGFR exists in over 60% of NSCLC cases and is recognized as an important target for cancer therapy. It has been found that the combination of tyrosine kinase inhibitors (EGFR-TKIs) and XAV939 or another tankyrase inhibitor AZ1366, could significantly inhibit canonical WNT pathway and EGFR protein phosphorylation, enhancing the sensitivity of EGFR-mutated drug resistant NSCLC cells to EGFR-TKI [139, 140]. Dong and colleagues found that 2,3,6-trisubstituted quinoxaline derivative (GDK100017), a novel small molecule inhibitor of the canonical pathway, could suppress the proliferation of NSCLC cells and enhance their sensitivity to radiotherapy by blocking WNT2-induced activation of canonical WNT pathway [61, 141, 142]. In addition, some small molecules also inhibit the canonical WNT pathway in other ways. ICG-001 is a selective inhibitor of the canonical WNT pathway, which competes against β-catenin to bind to the N-terminal region of CBP and reduces the transcriptional activation effect of β-catenin/TCF. Recently, ICG-001 was found to inhibit the proliferation and invasion of NSCLC cells in vitro by abolishing the upregulating effect of BCAT1 on the canonical WNT pathway [143]. Curcumin is a naturally occurring phenolic compound and found to inhibit the proliferation and invasion of NSCLC cells through metastasis-associated protein 1 (MTA1)-mediated inactivation of canonical WNT pathway [144]. 25-hydroxyprotopanaxadiol is a natural compound isolated from Panax ginseng, and its derivatives exert anti-cancer activities by inhibiting the canonical WNT pathway in NSCLC cells [145].
4.5 Activating the expression of negative regulators
Contrary to the upregulation of WNT activators, negative regulators in canonical WNT pathway are often downregulated in many malignancies. WIF-1 is a member of secreted FZD-related protein family and could suppress the activation of the canonical and noncanonical WNT pathways by competitively binding to WNTs. Some studies have found that both the transcription and translation levels of the WIF-1 gene were reduced in NSCLC tissues because of the hypermethylation in its promoter region [146, 147]. Interestingly, the anesthetic procaine and the antiarrhythmic procainamide, inhibitors of DNA methylation, were found to restore the expression of WIF-1 and ensuing inactivation of canonical WNT pathway in NSCLC cells, suggesting these two drugs have potential therapeutic effects on lung cancer [148]. Similarly, bisdemethoxycurcumin inhibited TGF-β1-induced EMT in highly metastatic lung cancer cells by upregulating expression of WIF-1 protein [149]. In addition, WIF-1 was found to induce the autophagy and apoptosis of NSCLC cells by inhibiting DVL2-mediated activation of canonical WNT and PI3K/AKT/mTOR pathways [150]. In summary, upregulating the expression of negative regulators in canonical WNT pathway is also a potential approach for treating lung cancer.
4.6 Inhibiting the drug resistance of NSCLC cells
Certain WNTs, such as WNT2b, WNT6 and WNT11, are more lowly expressed in the chemo-resistant lung cancer cells, and cumulative evidence suggests that the activation of canonical WNT pathways, partially attributed to the upregulation of some key oncoproteins, such as serine-arginine protein kinase 1 (SRPK1), ras-associated binding protein 25 (Rab25) and B cell lymphoma 9 (BCL9), contributes to the resistance of NSCLC cells to anti-cancer drugs such as cisplatin and the first-line EGFR-TKIs through inducing the expression of several genes involved in multi-drug resistance such as anti-apoptotic isoform of Bcl-xL, Oct4 and Nanog, and genes involved in the proliferation and maintenance of CSCs like CD44 and CD133 [151,152,153,154,155,156]. Therefore, the combination of inhibitors of canonical WNT pathway and current anti-NSCLC drugs would undoubtedly overcome all manner of resistance and improve the therapeutic effectiveness. For example, trifluoperazine, an antipsychotic agent, enhances the inhibitory of EGFR-TKIs and overcomes drug resistance in lung CSCs by blocking the canonical WNT pathway [151]. Moreover, Cai et al. showed antagonism of miR-128-3p could reverse the chemoresistance of highly malignant NSCLC cells by inhibiting canonical WNT signaling-induced CSC-like properties [157]. Recently, Yan et al. found that inhibiting the activity of DCLK1, a CSC marker, restored the sensitivity of EGFR-TKIs-resistant NSCLS cells through suppression of canonical WNT pathway and cancer stemness [158]. In addition, Wang et al. demonstrated a novel CK2 inhibitor, HY1-Pt, could reverse cisplatin-induced resistance by suppressing CSCs through canonical WNT pathway [159]. Interestingly, some natural compound such as garcinol was also found to inhibit CSC-like phenotypes via inactivation of canonical WNT pathway and STAT3 in NSCLC [160]. Generally, negative regulators of the canonical WNT pathway have a synergistic effect with anti-NSCLS drugs and are promising drug candidates in controlling drug resistant NSCLC cells.
Currently, the research on targeted therapies for lung cancer based on WNT pathways is in its infancy due to the complexity of these pathways. It still needs to deeply understand the mechanisms of disorders of WNT pathways in the pathogenesis of lung cancer and drug resistance, thus screening out reliable biomarkers for early clinical diagnosis and prognosis estimation of NSCLC and therapeutic targets for this disease. Moreover, it is necessary to strengthen the research on the WNT pathways in cancer immunotherapy.
5 Conclusion
WNTs are a class of secretory proteins that play important roles in embryonic development and tissue homeostasis in a paracrine or autocrine manner. However, the abnormal signal transduction of WNT pathways induced by the ectopic expression or dysfunction of WNTs is one culprit for the occurrence and progression of many human malignancies, including NSCLC. In this review, the changes and action mechanisms of human WNTs in the pathogenesis of NSCLC and related therapeutic strategies were discussed separately. Except for WNT5a, WNT7a, and WNT11, most WNTs exert a carcinogenic role by regulating the canonical and/or noncanonical pathways. Additionally, the role of some WNTs has never been elucidated in NSCLC. In summary, we hope this review will be helpful to gain a deeper understanding of the role of WNTs and mediated WNT pathways in the pathogenesis of NSCLC and arouse more researchers to develop WNTs-based therapeutic approaches for NSCLC.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1(1):49–52.
Stewart DJ. Tumor and host factors that may limit efficacy of chemotherapy in non-small cell and small cell lung cancer. Crit Rev Oncol Hematol. 2010;75(3):173–234.
Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with Advanced non–small-cell lung Cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–27.
Wood SL, Pernemalm M, Crosbie PA, Whetton AD. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. 2014;40(4):558–66.
Kozono DE, Stinchcombe TE, Salama JK, Bogart J, Petty WJ, Guarino MJ, et al. Veliparib in combination with carboplatin/paclitaxel-based chemoradiotherapy in patients with stage III non-small cell lung cancer. Lung Cancer. 2021;159:56–65.
Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–75.
Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18(1):42–51.
Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–19.
Ali AK, Watson DE. Pharmacovigilance assessment of immune-mediated reactions reported for checkpoint inhibitor cancer immunotherapies. Pharmacotherapy. 2017;37(11):1383–90.
da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–69.
Abe Y, Tanaka N. The hedgehog signaling networks in lung cancer: the mechanisms and roles in tumor progression and implications for cancer therapy. Biomed Res Int. 2016;2016:7969286.
Sparaneo A, Fabrizio FP, Muscarella LA. Nrf2 and notch signaling in lung cancer: near the crossroad. Oxid Med Cell Longev. 2016;2016:7316492.
Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S, et al. Notch signaling and EMT in non-small cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. 2014;7:87.
Bugter JM, Fenderico N, Maurice MM. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer. 2020. https://doi.org/10.1038/s41568-020-00307-z.
Rim EY, Clevers H, Nusse R. The Wnt pathway: from signaling mechanisms to synthetic modulators. Annu Rev Biochem. 2022. https://doi.org/10.1146/annurev-biochem-040320-103615.
Skronska-Wasek W, Gosens R, Konigshoff M, Baarsma HA. WNT receptor signalling in lung physiology and pathology. Pharmacol Ther. 2018;187:150–66.
Rapp J, Jaromi L, Kvell K, Miskei G, Pongracz JE. WNT signaling—lung cancer is no exception. Respir Res. 2017;18(1):167.
Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106(1):djt356.
Song Z, Wang H, Zhang S. Negative regulators of wnt signaling in non-small cell lung cancer: theoretical basis and therapeutic potency. Biomed Pharmacother. 2019;118:109336.
Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801.
Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307(5947):131–6.
Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11(24):3286–305.
Nie X, Liu H, Liu L, Wang YD, Chen WD. Emerging roles of wnt ligands in human colorectal cancer. Front Oncol. 2020;10:1341.
Dale TC. Signal transduction by the wnt family of ligands. Biochem J. 1998;329(Pt 2):209–23. (Pt 2)(.
You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan K-l, et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc–induced apoptosis. J Cell Biol. 2002;157(3):429–40.
Gatcliffe TA, Monk BJ, Planutis K, Holcombe RF. Wnt signaling in ovarian tumorigenesis. Int J Gynecol Cancer. 2008;18(5):954–62.
Tabnak P, Mafakheri A, Haji Emsailpoor Z, Kazemi T, Shekari N. Regulatory interplay between microRNAs and WNT pathway in glioma. Biomed Pharmacother. 2021;143:112187.
White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142(2):219–32.
Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60.
Lin SR, Mokgautsi N, Liu YN. Ras and Wnt interaction contribute in prostate cancer bone metastasis. Molecules. 2020;25(10):2380.
Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–9.
Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2 + pathways. Oncogene. 1999;18(55):7860–72.
Katoh M. WNT/PCP signaling pathway and human cancer (review). Oncol Rep. 2005;14(6):1583–8.
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275(5307):1784–7.
Zhao H, Ming T, Tang S, Ren S, Yang H, Liu M, et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. 2022;21(1):144.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–12.
Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–6.
Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61(16):6050–4.
Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, et al. Notch post-translationally regulates beta-catenin protein in stem and progenitor cells. Nat Cell Biol. 2011;13(10):1244–51.
Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, et al. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J Cell Biol. 2003;162(5):889–98.
Miete C, Solis GP, Koval A, Bruckner M, Katanaev VL, Behrens J, et al. Galphai2-induced conductin/axin2 condensates inhibit Wnt/beta-catenin signaling and suppress cancer growth. Nat Commun. 2022;13(1):674.
Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101(6):1345–56.
Xu T, Zeng Y, Shi L, Yang Q, Chen Y, Wu G, et al. Targeting NEK2 impairs oncogenesis and radioresistance via inhibiting the Wnt1/beta-catenin signaling pathway in cervical cancer. J Exp Clin Cancer Res. 2020;39(1):183.
Xu X, Sun P-L, Li J-Z, Jheon S, Lee C-T, Chung J-H. Aberrant Wnt1/β-catenin expression is an independent poor prognostic marker of non-small cell lung cancer after surgery. J Thorac Oncol. 2011;6(4):716–24.
Ben-Ze’ev A, Shtutman M, Zhurinsky J. The integration of cell adhesion with gene expression: the role of beta-catenin. Exp Cell Res. 2000;261(1):75–82.
Nakashima N, Huang CL, Liu D, Ueno M, Yokomise H. Intratumoral Wnt1 expression affects survivin gene expression in non-small cell lung cancer. Int J Oncol. 2010;37(3):687–94.
Gu B, Wang J, Song Y, Wang Q, Wu Q. microRNA-383 regulates cell viability and apoptosis by mediating Wnt/beta-catenin signaling pathway in non-small cell lung cancer. J Cell Biochem. 2018. https://doi.org/10.1002/jcb.28069.
Chen Y, Min L, Ren C, Xu X, Yang J, Sun X, et al. miRNA-148a serves as a prognostic factor and suppresses migration and invasion through Wnt1 in non-small cell lung cancer. PLoS ONE. 2017;12(2):e0171751.
Lin H, Li P, Zhang N, Cao L, Gao YF, Ping F. Long non-coding RNA MIR503HG serves as a tumor suppressor in non-small cell lung cancer mediated by wnt1. Eur Rev Med Pharmacol Sci. 2019;23(24):10818–26.
Li O, Kang J, Zhang JJ, Wang J, Hu LW, Li L, et al. Circle RNA FOXP1 promotes cell proliferation in lung cancer by regulating miR-185-5p/Wnt1 signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(12):6767–78.
Kerdidani D, Chouvardas P, Arjo AR, Giopanou I, Ntaliarda G, Guo YA, et al. Wnt1 silences chemokine genes in dendritic cells and induces adaptive immune resistance in lung adenocarcinoma. Nat Commun. 2019;10(1):1405.
Mehlman C, Takam Kamga P, Costantini A, Julie C, Dumenil C, Dumoulin J, et al. Baseline hedgehog pathway activation and increase of plasma Wnt1 protein are associated with resistance to immune checkpoint inhibitors in advanced non-small-cell lung cancer. Cancers. 2021;13(5):1107.
Li XF, Shen WZ, Jin X, Ren P, Zhang J. Let-7c regulated epithelial-mesenchymal transition leads to osimertinib resistance in NSCLC cells with EGFR T790M mutations. Sci Rep. 2020;10(1):11236.
You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, et al. Inhibition of wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23(36):6170–4.
Katoh M. Frequent up-regulation of WNT2 in primary gastric cancer and colorectal cancer. Int J Oncol. 2001;19(5):1003–7.
Cheng XX, Wang ZC, Chen XY, Sun Y, Kong QY, Liu J, et al. Correlation of Wnt-2 expression and beta-catenin intracellular accumulation in chinese gastric cancers: relevance with tumour dissemination. Cancer Lett. 2005;223(2):339–47.
Watanabe O, Imamura H, Shimizu T, Kinoshita J, Okabe T, Hirano A, et al. Expression of twist and wnt in human breast cancer. Anticancer Res. 2004;24(6):3851–6.
Huang C, Ma R, Xu Y, Li N, Li Z, Yue J, et al. Wnt2 promotes non-small cell lung cancer progression by activating WNT/β-catenin pathway. Am J Cancer Res. 2015;5(3):1032–46.
Bravo DT, Yang Y-L, Kuchenbecker K, Hung M-S, Xu Z, Jablons DM, et al. Frizzled-8 receptor is activated by the Wnt-2 ligand in non-small cell lung cancer. BMC Cancer. 2013;13:316.
Lee SB, Gong YD, Park YI, Dong MS. 2,3,6-Trisubstituted quinoxaline derivative, a small molecule inhibitor of the Wnt/beta-catenin signaling pathway, suppresses cell proliferation and enhances radiosensitivity in A549/Wnt2 cells. Biochem Biophys Res Commun. 2013;431(4):746–52.
Katoh M. Differential regulation of WNT2 and WNT2B expression in human cancer. Int J Mol Med. 2001;8(6):657–60.
Liu W, Zhang B, Xu N, Wang MJ, Liu Q. miR-326 regulates EMT and metastasis of endometrial cancer through targeting TWIST1. Eur Rev Med Pharmacol Sci. 2017;21(17):3787–93.
Wang B, Sun L, Li J, Jiang R. miR-577 suppresses cell proliferation and epithelial-mesenchymal transition by regulating the WNT2B mediated Wnt/beta-catenin pathway in non-small cell lung cancer. Mol Med Rep. 2018;18(3):2753–61.
Paiva I, Gil da Costa RM, Ribeiro J, Sousa H, Bastos M, Faustino-Rocha A, et al. A role for microRNA-155 expression in microenvironment associated to HPV-induced carcinogenesis in K14-HPV16 transgenic mice. PLoS ONE. 2015;10(1):e0116868.
Wang H, Fan L, Xia X, Rao Y, Ma Q, Yang J, et al. Silencing Wnt2B by siRNA interference inhibits metastasis and enhances chemotherapy sensitivity in ovarian cancer. Int J Gynecol Cancer. 2012;22(5):755–61.
Jiang H, Li F, He C, Wang X, Li Q, Gao H. Expression of Gli1 and Wnt2B correlates with progression and clinical outcome of pancreatic cancer. Int J Clin Exp Pathol. 2014;7(7):4531–8.
Kobayashi M, Huang CL, Sonobe M, Kikuchi R, Ishikawa M, Kitamura J, et al. Intratumoral Wnt2B expression affects tumor proliferation and survival in malignant pleural mesothelioma patients. Exp Ther Med. 2012;3(6):952–8.
Sumitomo R, Huang C-L, Ando H, Ishida T, Cho H, Date H. Wnt2b and Wnt5a expression is highly associated with M2 TAMs in non–small cell lung cancer. Oncol Rep. 2022;48(5):1.
Wu Y, Cheng K, Liang W, Wang X. lncRNA RPPH1 promotes non-small cell lung cancer progression through the miR-326/WNT2B axis. Oncol Lett. 2020;20(4):105.
Liu D, Kadota K, Ueno M, Nakashima N, Yokomise H, Huang CL. Adenoviral vector expressing short hairpin RNA targeting Wnt2B has an effective antitumour activity against Wnt2B2-overexpressing tumours. Eur J Cancer. 2012;48(8):1208–18.
Kobayashi M, Huang CL, Sonobe M, Kikuchi R, Date H. Ad-shWnt2b vector therapy demonstrates antitumor activity in orthotopic intrapleural models as monitored with the in vitro imaging system (IVIS). Anticancer Res. 2016;36(11):5887–93.
Katoh M. Molecular cloning and characterization of human WNT3. Int J Oncol. 2001;19(5):977–82.
Nambotin SB, Tomimaru Y, Merle P, Wands JR, Kim M. Functional consequences of WNT3/Frizzled7-mediated signaling in non-transformed hepatic cells. Oncogenesis. 2012;1(10):e31.
Wang HS, Nie X, Wu RB, Yuan HW, Ma YH, Liu XL, et al. Downregulation of human Wnt3 in gastric cancer suppresses cell proliferation and induces apoptosis. Onco Targets Ther. 2016;9:3849–60.
Kato S, Hayakawa Y, Sakurai H, Saiki I, Yokoyama S. Mesenchymal-transitioned cancer cells instigate the invasion of epithelial cancer cells through secretion of WNT3 and WNT5B. Cancer Sci. 2014;105(3):281–9.
Nakashima N, Liu D, Huang CL, Ueno M, Zhang X, Yokomise H. Wnt3 gene expression promotes tumor progression in non-small cell lung cancer. Lung Cancer. 2012;76(2):228–34.
Xing Z, Wang H-Y, Su W-Y, Liu Y-F, Wang X-X, Zhan P, Lv T-F. Wnt3 knockdown sensitizes human non-small cell type lung cancer (NSCLC) cells to cisplatin via regulating the cell proliferation and apoptosis. Eur Rev Med Pharmacol Sci. 2018. https://doi.org/10.26355/eurrev_201803_14474.
Nie X, Xia F, Liu Y, Zhou Y, Ye W, Hean P, et al. Downregulation of Wnt3 suppresses colorectal cancer development through inhibiting cell proliferation and migration. Front Pharmacol. 2019;10:1110.
Li C, Song G, Zhang S, Wang E, Cui Z. Wnt3a increases the metastatic potential of non-small cell lung cancer cells in vitro in part via its upregulation of Notch3. Oncol Rep. 2015;33(3):1207–14.
Song J-W, Zhu J, Wu X-X, Tu T, Huang J-Q, Chen G-Z, et al. GOLPH3/CKAP4 promotes metastasis and tumorigenicity by enhancing the secretion of exosomal WNT3A in non-small-cell lung cancer. Cell Death Dis. 2021;12(11):976.
Xu H, Jiao X, Wu Y, Li S, Cao L, Dong L. Exosomes derived from PM2.5–treated lung cancer cells promote the growth of lung cancer via the Wnt3a/beta–catenin pathway. Oncol Rep. 2019;41(2):1180–8.
Wang S, Qiu M, Xia W, Xu Y, Mao Q, Wang J, et al. Glypican-5 suppresses epithelial-mesenchymal transition of the lung adenocarcinoma by competitively binding to Wnt3a. Oncotarget. 2016;7(48):79736–46.
Li J, Lu R, Yang K, Sun Q. circCCT3 enhances invasion and epithelial-mesenchymal transition (EMT) of non-small-cell lung cancer (NSCLC) via the miR-107/Wnt/FGF7 axis. J Oncol. 2022;2022:7020774.
Prasad CP, Manchanda M, Mohapatra P, Andersson T. WNT5A as a therapeutic target in breast cancer. Cancer Metastasis Rev. 2018;37(4):767–78.
Asem MS, Buechler S, Wates RB, Miller DL, Stack MS. Wnt5a signaling in cancer. Cancers. 2016;8(9):79.
Bo H, Zhang S, Gao L, Chen Y, Zhang J, Chang X, et al. Upregulation of Wnt5a promotes epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells. BMC Cancer. 2013;13:496.
Lee GT, Kang DI, Ha YS, Jung YS, Chung J, Min K, et al. Prostate cancer bone metastases acquire resistance to androgen deprivation via WNT5A-mediated BMP-6 induction. Br J Cancer. 2014;110(6):1634–44.
Kanzawa M, Semba S, Hara S, Itoh T, Yokozaki H. WNT5A is a key regulator of the epithelial-mesenchymal transition and cancer stem cell properties in human gastric carcinoma cells. Pathobiology. 2013;80(5):235–44.
Jiang W, Crossman DK, Mitchell EH, Sohn P, Crowley MR, Serra R. WNT5A inhibits metastasis and alters splicing of Cd44 in breast cancer cells. PLoS ONE. 2013;8(3):e58329.
Bi L, Liu X, Wang C, Cao Y, Mao R, Li P, et al. Wnt5a involved in regulation of the biological behavior of hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7(3):987–95.
Yao L, Sun B, Zhao X, Zhao X, Gu Q, Dong X, et al. Overexpression of Wnt5a promotes angiogenesis in NSCLC. Biomed Res Int. 2014;2014:832562.
Whang YM, Jo U, Sung JS, Ju HJ, Kim HK, Park KH, et al. Wnt5a is associated with cigarette smoke-related lung carcinogenesis via protein kinase C. PLoS ONE. 2013;8(1):e53012.
Xi S, Xu H, Shan J, Tao Y, Hong JA, Inchauste S, et al. Cigarette smoke mediates epigenetic repression of miR-487b during pulmonary carcinogenesis. J Clin Invest. 2013;123(3):1241–61.
Huang CL, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H, et al. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor—an expression in non-small-cell lung cancer. J Clin Oncol. 2005;23(34):8765–73.
Wang B, Tang Z, Gong H, Zhu L, Liu X. Wnt5a promotes epithelial-to-mesenchymal transition and metastasis in non-small-cell lung cancer. Biosci Rep. 2017;37(6):BSR20171092.
Rapp J, Kiss E, Meggyes M, Szabo-Meleg E, Feller D, Smuk G, et al. Increased Wnt5a in squamous cell lung carcinoma inhibits endothelial cell motility. BMC Cancer. 2016;16(1):915.
Lu C, Wang X, Zhu H, Feng J, Ni S, Huang J. Over-expression of ROR2 and Wnt5a cooperatively correlates with unfavorable prognosis in patients with non-small cell lung cancer. Oncotarget. 2015;6(28):24912–21.
Huang Y, Liu G, Zhang B, Xu G, Xiong W, Yang H. Wnt-5a regulates proliferation in lung cancer cells. Oncol Rep. 2010;23(1):177–81.
Du W, Hu J, Hu R, Yang M, Peng Y, Zhang Z, et al. circ0101675 promotes malignant process via sponging miR-1278 and upregulating WNT3A/5A in non-small cell lung cancer. J Cancer. 2021;12(14):4209–17.
Zhao Y, Dai Q, Fu X, Chen Q, Tang Y, Gao X, et al. CircVAPA exerts oncogenic property in non-small cell lung cancer by the miR-876-5p/WNT5A axis. J Gene Med. 2021;23(6):e3325.
Chen TJ, Zheng Q, Gao F, Yang T, Ren H, Li Y, et al. MicroRNA-665 facilitates cell proliferation and represses apoptosis through modulating Wnt5a/beta-catenin and caspase-3 signaling pathways by targeting TRIM8 in LUSC. Cancer Cell Int. 2021;21(1):215.
Su G, Yan Z, Deng M. Sevoflurane inhibits proliferation, invasion, but enhances apoptosis of lung cancer cells by Wnt/beta-catenin signaling via regulating lncRNA PCAT6/miR-326 axis. Open Life Sci. 2020;15:159–72.
Guo W, Hu M, Wu J, Zhou A, Liao Y, Song H, et al. Gprc5a depletion enhances the risk of smoking-induced lung tumorigenesis and mortality. Biomed Pharmacother. 2019;114:108791.
Zhang L, Zeng S, Yu Z, Zhang G, Xiong Z, Xie F, et al. Overexpression of activating transcription factor-2 (ATF-2) activates Wnt/Ca2 + signaling pathways and promotes proliferation and invasion in non-small-cell lung cancer. Dis Markers. 2022;2022:1–10.
Yang J, Zhang K, Wu J, Shi J, Xue J, Li J, et al. Wnt5a increases properties of lung cancer stem cells and resistance to cisplatin through activation of Wnt5a/PKC signaling pathway. Stem Cells Int. 2016;2016:1–16.
Li H, Tong F, Meng R, Peng L, Wang J, Zhang R, et al. E2F1-mediated repression of WNT5A expression promotes brain metastasis dependent on the ERK1/2 pathway in EGFR-mutant non-small cell lung cancer. Cell Mol Life Sci. 2021;78(6):2877–91.
Wang SH, Chang JS, Hsiao JR, Yen YC, Jiang SS, Liu SH, et al. Tumour cell-derived WNT5B modulates in vitro lymphangiogenesis via induction of partial endothelial-mesenchymal transition of lymphatic endothelial cells. Oncogene. 2017;36(11):1503–15.
Yang L, Perez AA, Fujie S, Warden C, Li J, Wang Y, et al. Wnt modulates MCL1 to control cell survival in triple negative breast cancer. BMC Cancer. 2014;14:124.
Kikuchi A, Yamamoto H. Tumor formation due to abnormalities in the beta-catenin-independent pathway of Wnt signaling. Cancer Sci. 2008;99(2):202–8.
Zhang Q, Fan H, Liu H, Jin J, Zhu S, Zhou L, et al. WNT5B exerts oncogenic effects and is negatively regulated by mir-5587-3p in lung adenocarcinoma progression. Oncogene. 2020;39(7):1484–97.
Harada T, Yamamoto H, Kishida S, Kishida M, Awada C, Takao T, et al. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2017;108(1):42–52.
Ohira T, Gemmill RM, Ferguson K, Kusy S, Roche J, Brambilla E, et al. WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci USA. 2003;100(18):10429–34.
Bikkavilli RK, Avasarala S, Van Scoyk M, Arcaroli J, Brzezinski C, Zhang W, et al. Wnt7a is a novel inducer of β-catenin-independent tumor-suppressive cellular senescence in lung cancer. Oncogene. 2015;34(42):5317–28.
Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, et al. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005;280(20):19625–34.
Winn RA, Van Scoyk M, Hammond M, Rodriguez K, Crossno JT, Heasley LE, et al. Antitumorigenic effect of wnt 7a and fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281(37):26943–50.
Avasarala S, Bikkavilli RK, Van Scoyk M, Zhang W, Lapite A, Hostetter L, et al. Heterotrimeric G-protein, Galpha16, is a critical downstream effector of non-canonical wnt signaling and a potent inhibitor of transformed cell growth in non small cell lung cancer. PLoS ONE. 2013;8(10):e76895.
Avasarala S, Van Scoyk M, Wang J, Sechler M, Vandervest K, Brzezinski C, et al. hsa-miR29b, a critical downstream target of non-canonical wnt signaling, plays an anti-proliferative role in non-small cell lung cancer cells via targeting MDM2 expression. Biol Open. 2013;2(7):675–85.
Ji L, Minna JD, Roth JA. 3p21.3 tumor suppressor cluster: prospects for translational applications. Future Oncol. 2005;1(1):79–92.
Tennis MA, Vanscoyk MM, Wilson LA, Kelley N, Winn RA. Methylation of Wnt7a is modulated by DNMT1 and cigarette smoke condensate in non-small cell lung cancer. PLoS ONE. 2012;7(3):e32921.
Kim TH, Moon JY, Kim SH, Paik SS, Yoon HJ, Shin DH, et al. Clinical significance of aberrant Wnt7a promoter methylation in human non-small cell lung cancer in Koreans. J Korean Med Sci. 2015;30(2):155–61.
Xu X, Xu S, Wei Z, Li J. Wnt7a inhibits transformed cell proliferation while promoting migration and invasion in non-small cell lung cancer. Transl Cancer Res. 2020;9(8):4666–75.
Gao X-H, Zhang Y-L, Zhang Z-Y, Guo S-S, Chen X-B, Guo Y-Z. MicroRNA-96-5p represses breast cancer proliferation and invasion through Wnt/β-catenin signaling via targeting CTNND1. Sci Rep. 2020;10(1):44.
GorroÃąo-Etxebarria I, Aguirre U, Sanchez S, GonzÃĄlez N, Escobar A, Zabalza I, et al. Wnt-11 as a potential prognostic biomarker and therapeutic target in colorectal cancer. Cancers. 2019;11(7):908.
Murillo-Garzón V, Gorroño-Etxebarria I, Åkerfelt M, Puustinen MC, Sistonen L, Nees M, et al. Frizzled-8 integrates Wnt-11 and transforming growth factor-β signaling in prostate cancer. Nat Commun. 2018;9(1):1747.
Toyama T, Lee HC, Koga H, Wands JR, Kim M. Noncanonical Wnt11 inhibits hepatocellular carcinoma cell proliferation and migration. Mol Cancer Res. 2010;8(2):254–65.
Chellappan SP, Bartis D, Csongei V, Weich A, Kiss E, Barko S, et al. Down-regulation of canonical and up-regulation of non-canonical Wnt signalling in the carcinogenic process of squamous cell lung carcinoma. PLoS ONE. 2013;8(3):e57393.
Zeng F-R, Zhou X-Y, Zeng L-G, Sun J-C, He F, Mo W, et al. Identification of key genes and pathway related to chemoresistance of small cell lung cancer through an integrative bioinformatics analysis. Ann Transl Med. 2022;10(18):968.
Tenjin Y, Matsuura K, Kudoh S, Usuki S, Yamada T, Matsuo A, et al. Distinct transcriptional programs of SOX2 in different types of small cell lung cancers. Lab Invest. 2020;100(12):1575–88.
Tenjin Y, Kudoh S, Kubota S, Yamada T, Matsuo A, Sato Y, et al. Ascl1-induced Wnt11 regulates neuroendocrine differentiation, cell proliferation, and E-cadherin expression in small-cell lung cancer and Wnt11 regulates small-cell lung cancer biology. Lab Invest. 2019;99(11):1622–35.
Wang J, Yang Q, Tang M, Liu W. Validation and analysis of expression, prognosis and immune infiltration of WNT gene family in non-small cell lung cancer. Front Oncol. 2022;12:911316.
Gao L, Hu Y, Tian Y, Fan Z, Wang K, Li H, et al. Lung cancer deficient in the tumor suppressor GATA4 is sensitive to TGFBR1 inhibition. Nat Commun. 2019;10(1):1665.
Yang Y, Fan X, Nie Y, Liu D, Zhu D, Wu K, et al. CircTUBGCP3 facilitates the tumorigenesis of lung adenocarcinoma by sponging miR-885-3p. Cancer Cell Int. 2021;21(1):651.
Guimaraes PPG, Tan M, Tammela T, Wu K, Chung A, Oberli M, et al. Potent in vivo lung cancer wnt signaling inhibition via cyclodextrin-LGK974 inclusion complexes. J Control Release. 2018;290:75–87.
Shah K, Panchal S, Patel B. Porcupine inhibitors: novel and emerging anti-cancer therapeutics targeting the Wnt signaling pathway. Pharmacol Res. 2021;167:105532.
Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, et al. Wnt pathway inhibition via the targeting of frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109(29):11717–22.
Le PN, McDermott JD, Jimeno A. Targeting the wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11.
Li C, Zheng X, Han Y, Lv Y, Lan F, Zhao J. XAV939 inhibits the proliferation and migration of lung adenocarcinoma A549 cells through the WNT pathway. Oncol Lett. 2018;15(6):8973–82.
Togashi Y, Hayashi H, Terashima M, de Velasco MA, Sakai K, Fujita Y, et al. Inhibition of beta-catenin enhances the anticancer effect of irreversible EGFR-TKI in EGFR-mutated non-small-cell lung cancer with a T790M mutation. J Thorac Oncol. 2015;10(1):93–101.
Scarborough HA, Helfrich BA, Casas-Selves M, Schuller AG, Grosskurth SE, Kim J, et al. AZ1366: an inhibitor of tankyrase and the canonical Wnt pathway that limits the persistence of non-small cell lung cancer cells following EGFR inhibition. Clin Cancer Res. 2017;23(6):1531–41.
Lee SB, Park YI, Dong MS, Gong YD. Identification of 2,3,6-trisubstituted quinoxaline derivatives as a Wnt2/beta-catenin pathway inhibitor in non-small-cell lung cancer cell lines. Bioorg Med Chem Lett. 2010;20(19):5900–4.
Gong Y-D, Dong M-S, Lee S-B, Kim N, Bae M-S, Kang N-S. A novel 3-arylethynyl-substituted pyrido[2,3,-b]pyrazine derivatives and pharmacophore model as Wnt2/β-catenin pathway inhibitors in non-small-cell lung cancer cell lines. Bioorg Med Chem. 2011;19(18):5639–47.
Lin X, Tan S, Fu L, Dong Q. BCAT1 overexpression promotes proliferation, invasion, and Wnt signaling in non-small cell lung cancers. Onco Targets Ther. 2020;13:3583–94.
Lu Y, Wei C, Xi Z. Curcumin suppresses proliferation and invasion in non-small cell lung cancer by modulation of MTA1-mediated Wnt/beta-catenin pathway. In Vitro Cell Dev Biol Anim. 2014;50(9):840–50.
Bi X, Xia X, Mou T, Jiang B, Fan D, Wang P, et al. Anti-tumor activity of three ginsenoside derivatives in lung cancer is associated with Wnt/beta-catenin signaling inhibition. Eur J Pharmacol. 2014;742:145–52.
Tang Q, Zhao H, Yang B, Li L, Shi Q, Jiang C, et al. WIF-1 gene inhibition and wnt signal transduction pathway activation in NSCLC tumorigenesis. Oncol Lett. 2017;13(3):1183–8.
Mazieres J, He B, You L, Xu Z, Lee AY, Mikami I, et al. Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res. 2004;64(14):4717–20.
Gao Z, Xu Z, Hung MS, Lin YC, Wang T, Gong M, Jablons DM, et al. Procaine and procainamide inhibit the Wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells. Oncol Rep. 2009;22(06):1479–84.
Xu J-H, Yang H-P, Zhou X-D, Wang H-J, Gong L, Tang C-L. Role of Wnt inhibitory factor-1 in inhibition of bisdemethoxycurcumin mediated epithelial-to-mesenchymal transition in highly metastatic lung cancer 95D cells. Chin Med J. 2015;128(10):1376–83.
Luo X, Ye S, Jiang Q, Gong Y, Yuan Y, Hu X, et al. Wnt inhibitory factor-1-mediated autophagy inhibits Wnt/β-catenin signaling by downregulating dishevelled-2 expression in non-small cell lung cancer cells. Int J Oncol. 2018;53(2):904–14.
Yeh CT, Wu AT, Chang PM, Chen KY, Yang CN, Yang SC, et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am J Respir Crit Care Med. 2012;186(11):1180–8.
Guan S, Chen X, Chen Y, Xie W, Liang H, Zhu X, et al. FOXM1 variant contributes to gefitinib resistance via activating Wnt/beta-catenin signal pathway in patients with non-small cell lung cancer. Clin Cancer Res. 2022;28(17):3770–84.
Huang JQ, Duan LX, Liu QY, Li HF, Hu AP, Song JW, et al. Serine-arginine protein kinase 1 (SRPK1) promotes EGFR-TKI resistance by enhancing GSK3beta Ser9 autophosphorylation independent of its kinase activity in non-small-cell lung cancer. Oncogene. 2023;42(15):1233–46.
Wang J, Zhou P, Wang X, Yu Y, Zhu G, Zheng L, et al. Rab25 promotes erlotinib resistance by activating the beta1 integrin/AKT/beta-catenin pathway in NSCLC. Cell Prolif. 2019;52(3):e12592.
Liu L, Zhu H, Liao Y, Wu W, Liu L, Liu L, et al. Inhibition of Wnt/beta-catenin pathway reverses multi-drug resistance and EMT in Oct4(+)/Nanog(+) NSCLC cells. Biomed Pharmacother. 2020;127:110225.
Zhang Y, Zhang Q, Chen H, Wang C. BCL9 promotes epithelial mesenchymal transition and invasion in cisplatin resistant NSCLC cells via beta-catenin pathway. Life Sci. 2018;208:284–94.
Cai J, Fang L, Huang Y, Li R, Xu X, Hu Z, et al. Publisher correction: simultaneous overactivation of Wnt/beta-catenin and TGFbeta signalling by mir-128-3p confers chemoresistance-associated metastasis in NSCLC. Nat Commun. 2018;9:16196.
Yan R, Fan X, Xiao Z, Liu H, Huang X, Liu J, et al. Inhibition of DCLK1 sensitizes resistant lung adenocarcinomas to EGFR-TKI through suppression of Wnt/beta-catenin activity and cancer stemness. Cancer Lett. 2022;531:83–97.
Wang Y, Wang X, Xu G, Gou S. Novel CK2-Specific pt(II) compound reverses cisplatin-induced resistance by inhibiting cancer cell stemness and suppressing DNA damage repair in non-small cell lung cancer treatments. J Med Chem. 2021;64(7):4163–78.
Huang WC, Kuo KT, Adebayo BO, Wang CH, Chen YJ, Jin K, et al. Garcinol inhibits cancer stem cell-like phenotype via suppression of the Wnt/beta-catenin/STAT3 axis signalling pathway in human non-small cell lung carcinomas. J Nutr Biochem. 2018;54:140–50.
Yuan S, Yu Z, Liu Q, Zhang M, Xiang Y, Wu N, et al. GPC5, a novel epigenetically silenced tumor suppressor, inhibits tumor growth by suppressing Wnt/β-catenin signaling in lung adenocarcinoma. Oncogene. 2016;35(47):6120–31.
Tian XH, Hou WJ, Fang Y, Fan J, Tong H, Bai SL, et al. XAV939, a tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma cell lines by inhibiting Wnt/beta-catenin signaling pathway. J Exp Clin Cancer Res. 2013;32(1):100.
Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J, et al. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin Cancer Res. 2017;23(24):7490–7.
Wan Mohd Tajuddin WNB, Lajis NH, Abas F, Othman I, Naidu R. Mechanistic understanding of curcumin’s therapeutic effects in lung cancer. Nutrients. 2019;11(12):2989.
Funding
This work was supported by National Natural Science Foundation of China (81700731), the Natural Science Foundation of Henan Province (232300420048), and the Province and Ministry Co-construction Youth Program for Medical Science and Technology of Henan Province (SBGJ202103105) to X.N.; The Key Program for Science and Technology of Henan Province (222102310174) to D.Y.; The Province and Ministry Co-construction Key Program for Medical Science and Technology of Henan Province (SBGJ202102214) and the Key Program for Science and Technology of Kaifeng City (2203005) to Y.X. We apologize to colleagues, whose work could not be cited due to a lack of space.
Author information
Authors and Affiliations
Contributions
XN, WX and LC wrote the manuscript. SL, YH, and YW collected the data and revised the manuscript. DY, YX and XN designed and revised the manuscript, drew the figures and secured fundings for this work. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xue, W., Cai, L., Li, S. et al. WNT ligands in non-small cell lung cancer: from pathogenesis to clinical practice. Discov Onc 14, 136 (2023). https://doi.org/10.1007/s12672-023-00739-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00739-7