Abstract
We reported previously that bone morphogenetic protein 7 (BMP7) could induce epithelial-mesenchymal transition (EMT) in PC-3 prostate cancer cells grown in tissue culture plates. In this study, we examined BMP7-induced morphological and molecular expression changes that are characteristic of EMT using these cells under both two- (2D) and three-dimensional (3D) culture conditions. Filamentous outgrowths from spheroid structures that were formed from PC-3 cells in 3D cultures were strikingly evident when the spheroids were exposed to extracellular BMP7. This morphological change in 3D was accompanied by down-regulation of E-cadherin, which is an essential adhesion molecule for the integrity of epithelial phenotype. Invasiveness of the cancer cells was significantly enhanced with BMP7 treatment along with activation and up-regulation of proteases such as MMP1, MMP13, and urokinase plasminogen activator. Signal transduction of EMT conversion was examined by the use of certain pathway-specific inhibitors. Of the chemical inhibitors tested, inhibitors of PI3 kinase and Erk were found to suppress BMP-induced morphological changes both in 2D and 3D conditions. These results suggest that, besides the Smad signaling pathways, BMP-induced activation of PI3K and Erk contribute to EMT morphologic conversion of the PC-3 prostate cancer cells. Together, the results support the notion that the complexity of EMT may be better evaluated in terms of both spatial and temporal processes in 3D cell culture models that are physiologically more relevant than the cell growth in tissue culture plates.
Similar content being viewed by others
Introduction
Epithelial-mesenchymal transition (EMT) is a cellular trans-differentiation program that enables polarized, immotile epithelial cells to convert to motile mesenchymal cells. EMT is involved in different stages of embryonic development as well as various human diseases [1]. There is growing evidence that EMT contributes to tumor cell invasion and dissemination, the hallmarks of malignancy [1]. Several distinct transcription factors are known to be capable of inducing EMT program in epithelial cells. The list of such factors includes Snail, Slug, Twist1/2, Goosecoid, FOXC2, ZEB1, and ZEB2/Sip1 [2]. Of the multiple extracellular signals that can initiate EMT, TGF-β appears to be the best characterized inducers in cancer pathogenesis [3].
Recently, we reported that bone morphogenetic proteins, members of TGF-β superfamily, are potent to induce EMT to the PC-3 human prostate cancer cells [4]. In that study, we examined EMT conversion of the cells in the conventional 2-dimensional (2D) cultures. However, cells on tissue culture planes are subject to stress that is extremely different from a physiological condition. As reviewed by Rhee and Grinnell [5], cells in 2D culture are placed at huge mechanical stress, a million times stronger than that of soft tissue. Since mechanotransduction can profoundly affect cell morphology, cytoskeleton arrangement, cell–cell adhesion and migration, the properties critical to EMT phenomenon, an analysis in a matrix-based 3D culture was thought to be more representative of a patho-physiological condition than the conventional methods using tissue culture plates [6, 7].
Here, we employed a 3D culture model, in parallel of 2D culture, to address molecular signaling of BMP-induced EMT in PC-3 prostate cancer cells. We determined cellular morphology and organization during EMT conversion as well as assessed some of the parameters of EMT, including the loss of cell adhesion such as E-cadherin, activation of relevant transcription factors Twist and Slug, and increase of matrix protease activity such as MMP 1 and 13. PI3K/Akt activation is known to play an important role in the rearrangement of the cytoskeleton and cell migration in many physiological events including TGF-β1-induced EMT [8–10]. Both in 2D and ECM-embedded cultures, we demonstrated that PI3K/Akt signaling is involved in the BMP7-induced morphologic EMT in the PC-3 cells. The 3D model of EMT allowed visualization of the temporal and collective migration of the cancer cells from the organized epithelial structures. Such experimental cell research models should be useful for understanding the fundamental mechanisms of EMT in development and in tumors.
Materials and Methods
Materials
Recombinant human BMP7 protein was diluted in low serum medium (either 0.1% or 0.5%) at a concentration of 50 ng/mL. Recombinant human BMP2, BMP6, TGF-β1, and recombinant mouse Noggin were purchased from R&D and reconstructed in 4 mM HCl buffer containing 0.1% bovine serum albumin. The chemical inhibitor U0126 was bought from Cell Signaling and other inhibitors, LY249002, SP600125, and SB203580 from EMD Biosciences. Inhibitors were dissolved in dimethyl sulfoxide (DMSO) and added to the culture medium. HCl buffer and DMSO solution were used in all control experiments. Giemsa Stain Solution was purchased from Sigma-Aldrich.
3D Culture Method and Recovery of Cells from Embedded Culture
PC-3 prostate cancer cells were obtained from ATCC and maintained in DMEM media supplemented 10% of fetal bovine serum (FBS). Other prostatic cell lines (BPH-1, DU145, LNCaP, and C4-2B) were cultured as previously described [4] and cells were placed in low serum containing media, either 0.1% or 0.5%. 3D culture method was modified from that of Debnath et al. [6]. Growth Factor Reduced Matrigel Matrix (BD Biosciences) was solidified at 37°C and single cell suspensions were mixed with Matrigel at a final concentration of 2.5%. When spheroid structures reached at about 50 μm (generally by 6 to 7 days of culture), the culture medium was replaced with low serum medium with or without BMP, and then replenished every 3 to 4 days while cell morphology was monitored daily. Transmission light microscope and Spot Advanced Plus software were used to collect and analyze images of 2D or 3D cultures. Dispase was purchased from BD Biosciences and used for recovery of cells in 3D culture by following the manufacturer’s instruction.
Hematoxylin and Eosin Staining
Culture media in 3D cultures were discarded and solid Matrigel matrix containing cell structures was frozen in OCT compound (Sakura Fineteck USA). Sections were sliced at 8 μm thickness using Microm 505E Cyrostat. Cryosection of 3D structure was fixed in 4% PFA for 10 min, rinsed three times with PBS for 5 min each time. Then cell membranes were permeabilized in 70% ethanol for 2 min and washed 2 min in tap water. Rest of procedure was as described followed the method of Liao et al. [11].
Semi-quantitative and Quantitative PCR
RNA extraction, cDNA synthesis, and quantitative PCR were performed following protocol described previously [12]. The sequences of primers used for semi-quantitative are described in Table 1. 2-Delta Ct analysis was made to determine the differences in mRNA expression levels [13].
Immunoblot Assay
Immunoblot assays were perfomed as described previously [14]. Antibodies against Akt, Erk, p38 kinase, and their phosphorylated forms were purchased from Cell Signaling Technology. Anti-actin antibody was from Santa Cruz Biotechnology.
Immunofluorescence Staining
Frozen sections or intact 3D structures embedded in Matrigel matrix were used for staining. Specimens were washed with PBS twice, fixed with 4% paraformaldehyde (w/v) for 15 min, and permeabilized by 0.2% Triton X-100 for 10 min. Matrigel-embedded samples had an extra rinse with PBS buffer containing 100 mM glycine. The rest of staining process was carried out as described [6]. Mouse anti-human E-cadherin (Zymed) was used and mounting solution (Vector Labs) stained nuclei shortly before microscopic observation. Fluorescence images were obtained using Epifluorescence microscope (Leica), Spinning disk confocal microscope (PerkinElmer) or Multiphoton laser scanning confocal microscope (Zeiss LSM510). Velocity software (PerkinElmer) performed fluorescence images analysis including 3D image reconstruction.
Cell Invasion Assays
PC-3 cells were originally cultured with or without BMP7 in low serum media for 2, 4, and 6 days after a day in low serum media. Cells in 2D culture were trypsinized to prepare single cell suspension and 105 cells/8 μm pore-sized Cell Culture Insert (BD Biosciences) were loaded on top of a layer of Matrigel inside the chamber. Matrigel was diluted 1:9 in the culture media. Cells were allowed to migrate for 18 h and the migrated cells at the bottom of the cell culture insert were stained in Giemsa staining solution (Sigma-Aldrich) and the every cell with visible nucleus staining was counted.
Scratch Assay
Scratch assay was performed as described by Yang et al. [4] to determine BMP-induced cell motility in the cultures.
Statistical Analysis
All experiments were repeated for three or more independent trials. Statistical comparisons were made by an unpaired two-tailed t test.
Results
BMP7 Stimulates Filamentous Outgrowth from PC-3 Spheroids in 3D Cultures
We examined cellular morphology and multicellular organization of the PC-3 prostate cancer cells by using the 3D “on-top” culture method. The method consisted of two phases (Fig. 1a); full serum medium was used initially to allow cell proliferation and generation of 3D structures in the Matrigel matrix, and then the medium was replaced with low serum medium containing the added factor of interest. Low serum condition was used to minimize possible intervention by other growth factors present in fetal bovine serum, and, in fact, we did not observe any significant changes in the structures of the spheroids or the cells when maintained under the low serum condition. Cancer cells were plated on top of Matrigel matrices as suspension of single cells. Cells were found to undergo active divisions forming clusters which appeared to further progress to develop spheroids with an internal lumen (Fig. 1b). From daily microscopic evaluations and by propidium iodide staining of nuclei, we were assured that the 3D structures formed over the time period of observation were not derived from simple aggregation of the single cells (data not shown). The efficiency of spheroid formation in Matrigel was determined to be about 1~2% of input.
BMP7 induces filamentous outgrowth of spheroids in PC-3 cells but not in other prostatic cell lines. a Scheme of 3D culture method used in the study. Primarily, single cell suspension was loaded on top of extracellular matrix and allowed to form multicellular structures in 10% FBS supplemented media. When the multicellular structures reached at size of average 50 μm or more, culture media were replaced with low serum (either 0.1% or 0.5% FBS) media in presence or absence of BMP. The drawing represents morphological change of PC-3 cells in BMP-supplemented medium. b Upper panel Spheroid structures were developed from single cells of PC-3 in full serum condition. Middle and bottom panels Low serum conditions were given to cells without or with 50 ng/ml of BMP7. BMP7 stimulated morphological conversion, from spheroids to filamentous outgrowth, while cells in low serum condition maintained spheroids. Days in full serum culture and in low serum with or without BMP7 treatment were noted. c Higher concentration of BMP7 in culture medium rapidly converted cell morphology, and BMP antagonist Noggin successfully suppressed the structural change
While the spheroid structures could be maintained under the low serum condition for many days, induction of filamentous outgrowth from the structures could be readily seen within a short time period (2 to 3 days) of BMP7 treatment (Fig. 1b). Morphological changes noted in the cellular organoids were also visible in the scattered single cells (Fig. 1 in the Electronic Supplementary Material (ESM)). The cells started to bud out from spheroid structures and the strands of elongated cells further migrated out radially. Presence of higher concentrations of BMP in culture media (Fig. 1c) or extended culture period in BMP media facilitated EMT conversion to increased number of cells, similar to what was observed in 2D cultures (Fig. 2a in the ESM). That the effect was directly related to BMP activity was confirmed by adding Noggin, an inhibitor of BMP action. Filamentous outgrowth was robustly inhibited by use of 5 ug/ml recombinant Noggin (Fig. 1c). The effect of Noggin was also inhibitory to EMT-like transition that was induced by BMP7 to PC-3 cells in 2D cultures (Fig. 2b in the ESM). PC-3 cells over-expressing BMP7 displayed fibroblastoid shapes but turned to increased number of cells with more rounded epitheloid morphology in proportion to the increased concentration of Noggin added to the culture medium (Fig. 2c in the ESM). Morphologic conversions similar to those induced by BMP7 were also encountered when the PC-3 cells in 3D cultures were treated with BMP2 or BMP6 (100 ng/ml), but exposure to TGF-β1, as we reported earlier in 2D cultures [4], failed to induce filamentous morphology to the spheroids. It should be noted that we also attempted to grow a few other human prostatic cell lines in 3D culture condition. These cell lines included benign prostatic hyperplasia BPH-1 cells, and DU145, LNCaP, and C4-2B prostate cancer cells. While spheroid formation could be detected with each of these cell lines except LNCaP, none did respond to BMP7 treatment to any significant extent in terms of EMT transition or filamentous outgrowth under the experimental conditions used (Fig. 3 in the ESM).
BMP7 Modulates E-cadherin and Related Transcription Factors Implicated in EMT Induction
EMT is primarily observed as a morphologic change accompanied with loss of polarity and alteration of cell–cell interaction. Down-regulation of epithelial marker expression, most remarkably the decrease of E-cadherin level, is prominent. Enhanced motility and invasiveness are essential elements of EMT as well, along with up-regulation of secretory proteases. Suppression of E-cadherin is known to enhance invasion of various types of cancer cells [14]. Inversely, ectopic expression of E-cadherin in fibroblasts altered cell–cell adhesion and cell shape to that of epithelial cells [15]. Immunostaining of 3D structures indicated a reduction of E-cadherin expression in BMP7-treated cells (Fig. 2a). The change was more obvious at peripheries. Both mRNA and protein expressions of E-cadherin were significantly reduced in BMP7-treated cells (Fig. 2b).
BMP7 decreases expression of E-cadherin and affects expression of transcription factors induce EMT conversion. a Upper panel shows images of PC-3 spheroids in low serum condition and bottom panel are stellate cellular filaments outgrown by BMP7. Left Panel 3D structures were shown by transmission light microscopy. Empty hollow is visible in the spheroid in the upper-left panel. Elongated fibroblastoid morphology of PC-3 cells in the filamentous structure is obvious in the bottom left panel. Scale bar, 50 μm; magnification, ×200. Middle panel Frozen sections of 3D structures were visualized by hematoxylin and eosin staining. Magnification, ×200. Right panel Immunofluorescence staining of intact 3D structure embedded in Matrigel. Loss of E-cadherin was apparent in the filamentous structure induced by 3 days of BMP7 treatment, compared with the spheroids under low serum condition. 3D structure of spheroids and filaments outgrown from spheroids were confirmed through reconstitution of Z-stack images collected by confocal microscopy (data not shown). Magnification, ×260. b Semi-quantitative PCR and Western blot analysis showed that E-cadherin expression is significantly decreased in BMP7-treated PC-3 cancer cells. Low serum condition did not have a significant effect on the level of E-cadherin. c Expression of transcription factors that mediate induction of EMT phenotype were determined by semi-quantitative PCR. Up to 4 days of BMP7 treatment, twist and slug transcription was enhanced in cancer cells. In contrast, Sip1 and Snail expressions were decreased in BMP-treated cells
Various factors that mediate gene expression of EMT have been described [16–19]. Transcription factors, such as Twist, Snail, Slug, and Sip1 are known to down-regulate E-cadherin transcription and induce EMT phenotype. Ectopic over-expression of one or more of these transcription factors could enhance both EMT conversion and metastatic potential of cancer cells. Likewise, inhibition of these transcription factors could robustly suppress EMT [20, 21]. We investigated the expression of these transcription factors in cells undergoing EMT conversion from exposure to extracellular BMP7 (Fig. 2c). Twist and Slug transcription factors were found to be up-regulated in the PC-3 cells by BMP7, although interestingly, the expression of Sip1 and Snail transcription factors were noted to be decreased.
BMP7 Enhances Protease Activities and Cell Invasion
Enhanced cell invasion is another characteristic of EMT, which may play a role in early process of cancer metastasis. Invasion of cancer cells was significantly increased after 6 days of BMP7 treatment (Fig. 3a). Notably, PC-3 cells that successfully migrated to the bottom of cell culture inserts displayed elongated cell shapes and extended filopodia. The migrated cells appeared to display a relatively higher nuclear to cytoplasm ratio as compared to those which did not migrate, a phenotype attributed to aggressive cancer cells [22].
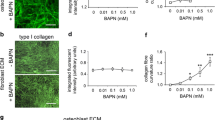
BMP7 increases invasion of PC-3 cancer cells, enhances protease activities, and upregulates protease expressions. a Number of invasive cells was counted using Matrigel invasion assays. 6 days of BMP treatment significantly enhanced cell invasiveness. *p < 0.05; **p < 0.001. b The activity of secretory proteases was evaluated using PC-3 conditioned media from cells previously cultured in low serum condition or in BMP-supplemented media for period indicated in graph legend. SensoLyte™520 MMP Substrate Sampler Kit was used. c Transcription of proteases was examined using quantitative PCR. Expression of each protease was normalized to the expression in low serum condition for 2 days of culture. 2-Delta Ct method was used to calculate mRNA change in folds and statistical assay (paired t test) was carried out to assure the statistically significant difference between BMP-treated and BMP-untreated samples. *p < 0.05; **p < 0.001
The secreted protease activity present in conditioned media (Fig. 3b) was assessed. Among MMP substrates that are included in SensoLyte™520 MMP Substrate Sampler Kit (AnaSpec), the activity to digest substrate 2, 6, and 10 was significantly increased in the conditioned medium of BMP-treated cells relative to that of the control cells. These substrates are indicative of the presence of the activity of MMP1/7/8/12/13, MMP2/13, and MMP13, respectively. It was noted that the extracellular activities targeting these substrates gradually increased with the culture period, both in low serum or low serum plus BMP7 condition.
The cell cultures were analyzed for the expression levels of specific protease genes. Transcription of MMP1 (interstitial collagenase), MMP13 (collagenase 3) and urokinase type-plasminogen activator (uPA) was increased by BMP7 by 3-, 2-, and 2.5-fold, respectively (Fig. 3c). In contrast, MMP9 transcription was slightly decreased by BMP7. The transcription of MMP3, MMP10 and MMP12 also appeared to be up-regulated by BMP7 but not in a statistically significant manner.
BMP7-induced Cell Migration Is Mediated by Akt and Erk Signal Transduction
PI3K/Akt activation plays an important role in the rearrangement of the cytoskeleton and cell migration in many physiological events including TGF-β1-induced EMT [8, 9]. Signal transduction of EMT is also mediated by Smad pathway, which is a canonical signaling pathway of TGF-β1 superfamily. We examined Akt pathway and the downstream Smad and MAPK pathways in BMP-induced EMT. Scratch assay was used to examine cell motility. Migration of cells to the wounded region was found to be accelerated by 6 ng/ml or higher concentration of BMP7 in low serum without affecting cell proliferation (Fig. 4a; Fig. 4a in the ESM).
Inhibition of Akt and Erk pathways decreases cell migration of BMP-activated cells. Scratch assays were performed to compare motility of cells in low serum, with BMP7 treatment or in combination of BMP7 and various kinase inhibitors. a A 6 ng/ml or higher concentration of BMP7 stimulated cell migration compared to low serum control. b Use of LY294002, a PI3K/Akt inhibitor, counteracted the BMP7 effect on both cell migration and morphology. c Chemical inhibitor of MEK1/2, U0126 that inhibits Erk phosphorylation and activation, significantly reduced migration of cells in BMP7 culture. d Activation of signal transduction of BMP was examined by Western blot analysis. Akt phosphorylation was increased in BMP-treated cells and Erk activation was shown after 6 days of culture
Chemical inhibitors of each signaling pathways was added in media containing BMP to inhibit EMT phenotype. LY249002 at 5 to 20 μM was able to offset BMP effect on cell migration without significant cytotoxicity, but the use of inhibitor at 40 μM concentration caused significant cell apoptosis (Fig. 4b; Fig. 4b in the ESM). PI3 kinase inhibitor affected cell shape as well; overall cell morphology became rounder compared to the corresponding control cells. Inhibition of BMP-Smad signaling by transfection of dominant negative Smad5-induced cell apoptosis as well.
The inhibition of Erk reduced the increased motility that was induced by BMP7 (Fig. 4c; Fig. 4c in the ESM), and subsequently led to cell apoptosis indicated by rounded cell shape, detachment from the surface and typical appearance under transmission light microscopy. Implication of JNK in BMP-induced EMT could not be determined by scratch assay because of excessive cell death from the exposure to JNK inhibitor (data not shown). Activation of Akt signaling was confirmed by Western blot assays. Akt expression and phosphorylation were increased by BMP7 (Fig. 4d). Activity of Erk pathway was up-regulated after 6 days of culture in BMP7-containing media, while p38 MAPK pathway was not affected.
Akt and Erk Signal Transduction also Regulates the Morphologic Change Induced by BMP7 in 3D Culture
Next, we examined whether the above signaling pathways might also regulate the morphological change in 3D culture induced by BMP. PI3 kinase inhibitor, LY249002 at 5 to 20 μM, inhibited filamentous outgrowth of spheroids that was observed in BMP7-treated cells (Fig. 5a). Erk inhibitor reduced spheroid budding at 5 μM or higher concentration, without causing cell death unlike what was detected in the scratch assays (Fig. 5b). Due to technical difficulties in performing transfection of matrix-embedded cells, small molecule inhibitor of BMP-Smad signaling was used. Dorsomorphin was the first known chemical inhibitor that binds to BMP receptor and specifically inhibit BMP-Smad signal transduction without affecting TGF-β-Smad signaling [23]. Inhibition of Smad signaling using dorsomorphin caused cell death at all concentration tested, 5 to 20 μM. Inhibitors of JNK or p38 kinase did not reduce BMP-induced EMT morphologic phenotype (Fig. 5c).
Akt and Erk pathways mediate morphological change of EMT induced by BMP7 in 3D. a Inhibition of PI3K/Akt effectively prevented the filamentous structure formation causes by BMP7, at all concentration of inhibitor used, 5 to 20 μM/ml. b Erk inhibitor was able to suppress the morphologic change at 10 μM concentration but the effect was less obvious at a lower concentration, 1 and 5 μM. c Dorsomorphin, specific chemical inhibitor of BMP-Smad signaling rather induced apoptosis in cells in 3D culture. d Both JNK inhibitor SP600125 and p38 kinase inhibitor SB203580 did not inhibit the BMP7 effect on 3D structure
Discussion
One of the biggest challenges of cancer treatment is the fatal metastasis to distal organs from the primary carcinoma in situ. EMT conversion has been observed in various cancer cells on tissue culture dishes and its connection to metastasis has been widely implicated, although limitations do exist. Microscopic observation of cell morphology is often subjective and thereby, may not always be sufficient to confirm EMT occurrence. There is no objective standard of EMT morphologic change and occasionally it is not obvious due to mixed morphology of the cells. Absence of extracellular matrix in cultures in vitro may also lead to misinterpretation of the phenotype. Furthermore, there is only a limited amount of evidence on how and to what extent EMT may play a role in cancer progression in vivo. Foci undergoing EMT are usually hard to find in histopathological screening of human tumor specimens and there are technical difficulties in confirming EMT conversions even when detected. It is recognized that better development of in vitro models of metastasis that allow both temporal and spatial analyses of the events of metastatic progression should be valuable. In this context, we employed the 3D “on-top” culture model to characterize BMP7-induced EMT in a prostate cancer cell line that we previously reported to be susceptible to such morphological changes in 2D cultures.
Ductal morphogenesis of prostate cancer cells was described in Matrigel cultures in vitro [24, 25]. By using 3D cultures, we demonstrate that the PC-3 prostatic epithelial cells are capable of forming organized glandular structures. The BMP effect on this 3D morphology leads to filamentous, stellate outgrowth. Individual cells in spheroids become extended migrating outwards while also generally maintaining cell–cell association. It has been hypothesized that movement of cancer cells during metastatic process is collaborative or collective, in the form of clusters, sheets, or lines of cells, rather than a migration of single cells [26, 27]. In the 3D model of EMT, we also observe this collective migration of prostate cancer cells that display a distinctive morphologic switch from epitheloid to fibroblastoid types.
We compared various features of EMT between PC-3 cancer cells cultured on tissue culture plate and cells in 3D matrix. In both in 2D and 3D conditions, BMP2, BMP6, and BMP7 exert the mesenchymal-like transition to PC-3 cancer cells while TGF-β1 does not have significant effect under either condition. Down-regulation of E-cadherin, increased expression of protease, enhanced cell invasion, and up-regulation of key transcription factors, Twist and Slug are found to be associated with the EMT conversion of the PC-3 cells. A temporal regulation of EMT conversion is also noted. Morphologic changes, increased cell motility and cell invasion become prominent after 6 days of treatment in 2D conditions. Enhanced cell motility is maintained even after BMP7 is withdrawn after 6 days. BMP7 transactivation of Twist and Slug is continuously up-regulated until 4 days of culture, but the increment turns slow at 6 days. Invasive potential of BMP-treated cells is significantly increased after 6 days of culture. These observations may imply that there is a temporal commitment to EMT conversion between 4 and 6 days of culture in 2D, a point which awaits further investigation.
Akt signaling pathways appear to mediate the phenotypic conversion, in both 2D and 3D cultures with BMP treatment. Activation of Akt signaling by BMP2 has been reported to enhance migration of prostate cancer cells [28]. It appears that Erk may also play a role in EMT signaling in the prostate cancer cells, although potentially in a secondary manner. While Akt expression and phosphorylation occurs quickly by BMP7, Erk activation is moderate and happens later in culture. Signal transduction of TGF-β via PI3K/Akt and Erk has been implicated in cancer cells [8, 9, 29–32] and here, we also implicate these pathways as important in BMP-induced EMT in prostate cancer PC-3 cells. Since a major aspect of BMP signal transduction is mediated by Smad phosphorylation, we also targeted BMP-Smad axis to prevent EMT induction. However, use of dominant negative Smad5 or chemical inhibitor of BMP type I receptors, dorsomorphin caused cell apoptosis that interfered with EMT analysis. It is very likely that BMP-Smad activation confers anti-apoptotic protection to PC-3 prostate cancer cells as we previously described for other prostate cancer cell lines [12, 33]. Survivin up-regulation by BMP-Smad signaling may be a common moderator of anti-apoptotic protection in prostate cancer cells. In this regard, Smad signaling pathway offers an important potential target for cancer [34–36].
It is to be noted that of the prostate cancer cell lines we tested only PC-3 cells were significantly sensitive to BMP7-induced EMT. A recent report from another laboratory describes some degree of sensitivity of PC-3 and more so for its malignant variant PC-3M to undergo spontaneous EMT-like conversion after 5 to 13 days in 3D culture in 2% serum containing medium [10]. There are reports of EMT induction to other human prostate epithelial cells. For example, tumorigenic sublines of BPH-1 but not the parental non-malignant BPH-1 line could be induced to undergo EMT upon stimulation by TGF-β [37]. Non-malignant BPH-1 cells with over-expression of snail, however, were shown to assume EMT and invasive phenotype in 3D cultures [38]. While our data clearly documents sensitivity of PC-3 cells to EMT induction by BMP7, a previous study described BMP7 as a potent inhibitor of TGF-β-induced EMT in an aggressive variant of PC-3 cells, PC-3M-Pro4 [39]. The PC-3-Pro4 cells have a complex origin. This cell line was derived from the PC-3M cell line that was isolated from liver metastases produced in nude mice subsequent to intrasplenic injection of PC-3 cells; PC-3M cells were injected into athymic mouse prostates for selection of variants with increasing metastatic potential [40]. A variant line that resulted from several rounds of reinjecting cells from xenograft tumors back into the mouse prostates was named PC-3M-Pro4. The increasingly higher tumorigenic and metastatic potential of the PC-3, PC-3M, and PC-3M-Pro4 cells in that order also parallels gradual loss of expression of epithelial markers, such as, E-cadherin, and acquisition of mesenchymal markers [40]. It is reported that in PC-3M-Pro4 cells BMP7 counteracts binding of Smad 3/4 complexes on CAGA boxes that is induced by TGF-β [39]. The differing response of PC-3 and PC-3M-Pro4 cells to BMP7 may thus be another example of cell type-specific or context-dependent effect of BMPs in prostate cancer that is widely recognized [41]. Considering the extensive cellular heterogeneity inherent in prostate cancer, sensitivity of even a minority of prostate cancer cells to EMT may be biologically highly meaningful in prostate tumor progression and metastasis.
In summary, this study validates EMT conversion, both from the analyses of morphological changes and characteristic molecular expressions in PC-3 prostate cancer cells that are induced by BMP7. Since cancer cells reside in a three-dimensional tissue environment, the 3D experimental cell model for prostate cancer should serve as a useful system for mechanistic analysis of EMT. Using the PC-3 experimental cell model, we document that important spatial and temporal information of the EMT process could be readily obtained. Besides the expected involvement of the BMP-Smad signaling, we show that activation of PI3K and Erk by BMP contributes to morphologic EMT induction that is associated with filamentous outgrowth from the multicellular spheroids.
References
Thiery JP et al (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Weinberg RA (2008) Twisted epithelial-mesenchymal transition blocks senescence. Nat Cell Biol 10(9):1021–1023
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9(4):265–273
Yang S et al (2005) Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res 65(13):5769–5777
Rhee S, Grinnell F (2007) Fibroblast mechanics in 3D collagen matrices. Adv Drug Deliv Rev 59(13):1299–1305
Debnath J, Muthuswamy SK, Brugge JS (2003) Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30(3):256–268
Li Q et al (2011) 3D models of epithelial-mesenchymal transition in breast cancer metastasis: high-throughput screening assay development, validation, and pilot screen. J Biomol Screen 16(2):141–154
Larue L, Bellacosa A (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24(50):7443–7454
Bakin AV et al (2000) Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275(47):36803–36810
Harma V et al (2010) A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One 5(5):e10431
Liao CP et al (2010) Mouse prostate cancer cell lines established from primary and post-castration recurrent tumors. Horm Cancer 1(1):44–54
Lim M et al (2009) Runx2 regulates survivin expression in prostate cancer cells. Lab Invest 90:222–233
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408
Vleminckx K et al (1991) Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66(1):107–119
Nagafuchi A et al (1987) Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature 329(6137):341–343
Kang Y, Massague J (2004) Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118(3):277–279
Yang J et al (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117(7):927–939
Martin TA et al (2005) Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol 12(6):488–496
Zavadil J, Bottinger EP (2005) TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24(37):5764–5774
Comijn J et al (2001) The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 7(6):1267–1278
Kwok WK et al (2005) Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res 65(12):5153–5162
Isaacs, WB (ed) (2007) Prostate cancer: biology, genetics, and the new therapeutics. Humana Press, Totowa
Yu PB et al (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4(1):33–41
Lang SH et al (2001) Prostate epithelial cell lines form spheroids with evidence of glandular differentiation in three-dimensional Matrigel cultures. Br J Cancer 85(4):590–599
Lang SH et al (2001) Experimental prostate epithelial morphogenesis in response to stroma and three-dimensional matrigel culture. Cell Growth Differ 12(12):631–640
Friedl P, Gilmour D (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10(7):445–457
Giampieri S et al (2009) Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 11(11):1287–1296
Lai TH et al (2008) Osteoblasts-derived BMP-2 enhances the motility of prostate cancer cells via activation of integrins. Prostate 68(12):1341–1353
Grille SJ et al (2003) The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res 63(9):2172–2178
Xie L et al (2004) Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia 6(5):603–610
Bhowmick NA et al (2001) Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell 12(1):27–36
Xie L et al (2003) Transforming growth factor beta-regulated gene expression in a mouse mammary gland epithelial cell line. Breast Cancer Res 5(6):R187–R198
Akech J et al (2010) Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene 29(6):811–821
Miyaki M et al (1999) Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene 18(20):3098–3103
Colland F et al (2004) Functional proteomics mapping of a human signaling pathway. Genome Res 14(7):1324–1332
Katsuno Y et al (2008) Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 27(49):6322–6333
Ao M et al (2006) Transforming growth factor-beta promotes invasion in tumorigenic but not in nontumorigenic human prostatic epithelial cells. Cancer Res 66(16):8007–8016
Chu JH et al (2009) Development of a three-dimensional culture model of prostatic epithelial cells and its use for the study of epithelial-mesenchymal transition and inhibition of PI3K pathway in prostate cancer. Prostate 69(4):428–442
Buijs JT et al (2007) BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am J Pathol 171(3):1047–1057
Pettaway CA et al (1996) Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res 2(9):1627–1636
Morrissey C et al (2010) Bone morphogenetic protein 7 is expressed in prostate cancer metastases and its effects on prostate tumor cells depend on cell phenotype and the tumor microenvironment. Neoplasia 12(2):192–205
Acknowledgments
We thank Dr. A. Hari Reddi of UC Davis Medical Center for the generous supply of recombinant BMP7, and all members of the Roy-Burman laboratory for their support in various aspects of this study. This work was supported by grants from the National Institutes of Health: RO1 CA59705 and RO1 CA113392 (to P.R-B.).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PPTX 1549 kb)
Rights and permissions
About this article
Cite this article
Lim, M., Chuong, CM. & Roy-Burman, P. PI3K, Erk Signaling in BMP7-Induced Epithelial-Mesenchymal Transition (EMT) of PC-3 Prostate Cancer Cells in 2- and 3-Dimensional Cultures. HORM CANC 2, 298 (2011). https://doi.org/10.1007/s12672-011-0084-4
Published:
DOI: https://doi.org/10.1007/s12672-011-0084-4