Abstract
The trichorhinophalangeal syndrome 1 (TRPS-1) gene is a novel GATA transcription factor family member. Previously, using a gene expression profiling and immunohistochemistry (IHC) screen, we identified TRPS-1 as a highly prevalent gene in breast cancer (BC), expressed in >90% of estrogen receptor alpha (ERα)+ and ERα− BC subtypes. TRPS-1 was also shown to be expressed in prostate cancer where it was shown to play a proapoptotic function during androgen withdrawal possibly through regulating antioxidant metabolism. The role of TRPS-1 and its prognostic significance in hormone-dependent and hormone-independent BC however is not known. In this study, we developed a new quantitative IHC (qIHC) method to further study TRPS-1 as a marker and possible prognostic indicator in BC. By using this method, a quantitative parameter for TRPS-1 expression called a quick score (QS) was derived from the measured labeling index and mean optical density after IHC and applied to a set of 152 stage II/III BC patients from 1993 to 2006 who did not receive preoperative chemotherapy. Associations between QS and tumor characteristics were evaluated using the Kruskal–Wallis test. A wide range of TRPS-1 QS was found among the sample set with higher TRPS-1 QS significantly associated with tumor ERα (p = 0.023 for QS and p = 0.028 for Allred score), progesterone receptor (p = 0.009), and GATA-3 (p < 0.0001). TRPS-1 QS was also positively associated with HER2 status (p = 0.026). Further analysis of different ductal structures in ten BC cases revealed that TRPS-1 expression was expressed at low levels in the remaining normal ducts and in areas of usual ductal hyperplasia but showed marked increase in expression in ductal carcinoma in situ and invasive carcinoma lesions in the tissue. An analysis of TRPS-1 expression in association with overall survival in the 152 stage II/III sample set also revealed that TRPS-1 QS (≥4.0) was significantly associated with improved survival (p = 0.0165). Patients with TRPS-1 QS <4 had a hazard ratio of 2 (p = 0.019) after univariate Cox proportional hazards analysis. In summary, this new qIHC approach was found to reveal critical differences in TRPS-1 expression in primary BC samples and found that it is a promising prognostic marker that should be further evaluated as a possible tumor suppressor gene facilitating improved survival in different subtypes of BC.
Similar content being viewed by others
Introduction
Human breast cancer (BC) is the most common type of cancer among women in the USA. The initiation and progression of BC are affected by the contribution of many signaling pathways. In this regard, identification of new genes showing specific expression and high prevalence in BC may not only lead to the discovery of new prognostic markers but may also uncover new molecular pathways regulating BC cell survival and apoptosis that can be targeted for cancer treatment. Recently, the GATA transcription factor family, such as GATA-3, has been found to be necessary for mammary gland morphogenesis, luminal cell differentiation, proliferation, and maintenance [1, 8, 23]. GATA-3 also plays an important role in estrogen signaling driving BC progression and survival [1, 8, 23]. Thus, factors modulating the extent of GATA transcription factor activity would also be predicted to play a critical role in BC. Recently, we performed a comprehensive microarray-based differential gene expression analysis on RNA isolated from over 60 breast tumors of varying histologies in comparison to over 200 normal tissue RNA samples [20]. Using this approach, we identified a GATA family member called trichorhinophalangeal syndrome type 1 (TRPS-1) as being overexpressed in BC with little or no expression in normal tissues. We developed and validated a monoclonal antibody (mAb) against TRPS-1 and found that TRPS-1 was expressed at the protein level in >90% of all BC, including ductal carcinoma in situ (DCIS) and invasive ductal and lobular carcinomas [20].
TRPS-1 was initially identified not in cancer but as the gene associated with three rare autosomal dominant syndromes called trichorhinophalangeal (TRP) syndrome [15] characterized by changes in hair distribution and other facial developmental defects including a bulbous nose, a long and flat philtrum, and a thin upper lip [17]. TRP syndrome is associated with loss-of-function mutations in the TRPS-1 gene [14, 15]. However, gain of function may also have pathological consequences. Sequence analysis showed that TRPS-1 is composed of 1,294 amino acids, consisting of seven classical C2H2-type zinc finger domains and two Ikaros C2H2-type zinc finger domains. Moreover, TRPS-1 contains a single GATA C4-type zinc finger domain, also present in transcription factors belonging to the GATA family [17]; this places it in the GATA transcription factor family as a new member [16]. However, molecular analysis in vitro has shown that it plays a transcriptional suppressive rather than activating role by binding to GATA elements upstream of GATA-dependent genes in coinhibitory complexes and preventing GATA-induced transcription [16, 17, 19]. Interestingly, TRPS-1 was also found to be expressed in androgen-dependent prostate cancer, where it may inhibit androgen receptor signaling driving genes such as prostate-specific antigen (PSA) [25] and promote apoptosis after androgen withdrawal [5, 6, 21]. Moreover, TRPS-1 has also been found to regulate apoptosis sensitivity in chondrocytes by modulating STAT3 expression, a GATA-3-induced gene. Thus, TRPS-1 seems to be emerging as an important tumor suppressor. However, its role in BC initiation and progression and whether it has a suppressive role by regulating estrogen receptor (ER) signaling and apoptosis sensitivity is not known. Moreover, how TRPS-1 is expressed relative to ER, progesterone receptor (PR), GATA-3, and HER2 (markers for the major subsets of BC) is not known. Given the high prevalence of the gene in BC in our initial screening, we hypothesize that it plays a critical role in hormone-dependent BC and may be a prognostic marker related to ERα and GATA-3 expression.
In this study, we developed and optimized a quantitative IHC (qIHC) method to measure TRPS-1 protein in stained formalin-fixed, paraffin-embedded breast cancer samples from a cohort of stage II/III BC patients in order to evaluate the ability of reliably performing qIHC studies for TRPS-1 as well as evaluate interactions with other tumor characteristics and patient clinical performance. We also evaluated the possible prognostic significance of TRPS-1 using this qIHC method by performing an overall survival time analysis post-primary-surgery relative to quantitative level of TRPS-1 expression.
Materials and Methods
Patients and Tissue Samples
The study subjects were female patients with a history of either stage II or stage III breast carcinoma, diagnosed between 1993 and 2006, and who did not receive neoadjuvant chemotherapy and were selected from a clinical database at UT M.D. Anderson Cancer Center (UTMDACC) and the Veterans Administration Hospital, Baylor College of Medicine (VAH). The institutional review boards (IRB) of UTMDACC and the VAH approved the retrospective review of the medical records and identification and analysis of tumor blocks for the purposes of the current study. One hundred and fifty-two tumor blocks were identified. Follow-up time among patients who were alive at the end of follow-up ranged from 10 days to 18.15 years (median = 4.78 years). Patients who died during the study period were followed from 71 days to 9.6 years (median = 3.85 years) following the date of surgery. Breast cancer diagnosis was made by core needle or excisional biopsy of the breast tumor. All pathologic specimens were reviewed by dedicated breast pathologists. The histological type of the tumor specimens was defined according to the World Health Organization’s Classification System [26]. The histological grade was defined according to the modified Black’s nuclear grading system [4]. We also obtained 11 cases of previously diagnosed stage II/III male invasive ductal carcinoma from the VAH for an exploratory study to determine the extent of TRPS-1 expression in male BC. These cases were also collected under an approved IRB protocol. Among 152 stage II/III female BC, we randomly selected ten cases which had different histological regions, including adjacent normal ducts, hyperplasia, and ductal carcinoma in situ, to analyze how TRPS-1 was expressed in these regions.
The ovarian cancer, endometrial cancer, and colorectal cancer tissue microarrays (TMA) were acquired from Cybrdi, Inc. (Rockville, MD, USA). Each case had 1–3 cores of 1.5 mm in diameter and was cut from representative samples confirmed by pathologists. The lung cancer TMA was acquired from UTMDACC under an approved IRB protocol. Each lung cancer case had three cores of 0.6 mm in diameter each and was cut from a primary tumor identified and confirmed by pathologists.
Immunohistochemistry
mAb against human TRPS-1 was generated in mice and reactive clones isolated and screened previously [20]. Two hybridoma clones (8D11 and 8A1) that had comparable activity were picked for purification and use in IHC studies. The 8D11 clone was tested and validated for specificity using IHC on breast cancer and normal tissue samples, immunoblot analysis on breast cancer cell lines, and immunofluorescence staining as described in a previous publication [20]. Five-micrometer paraffin-fixed tissue sections were deparaffinized in xylene and rehydrated through graded alcohols (100%, 95% to 80%). Antigen retrieval was carried out for 30 min using PT Module (Lab Vision Corporation, USA) in Tris–EDTA buffer (pH 9.0). After cooling down, the slides were thoroughly washed in distilled water and washed three times in 1× phosphate-buffered saline (PBS), 2 min each. Endogenous peroxidase activity was quenched by immersion in 3% hydrogen peroxide (Sigma), then in methanol for 10 min at room temperature followed by rinsing for 2 min in 1× PBS three times. Nonspecific binding of the primary antibody was blocked by incubating the sections with 10% normal horse serum for 30 min at room temperature. Sections were then incubated with primary anti-TRPS-1 mAb clone 8D11 (1:1,000), anti-ERα mAb clone 1D5 (1:1,000, Dako, Carpinteria, CA), anti-HER2 mAb clone CB11 (1:150, Invitrogen, Carlsbad, CA, USA), or anti-GATA3 mAb HG3-31 (1:450, Santa Cruz Biotechnology, CA, USA) at 4°C overnight. The second day, after washing three times in 1× PBS (2 min each), the slides were incubated with secondary antimouse IgG-biotin antibody (1:200, Vectastain Elite ABC Kit; Vector laboratories, CA, USA) at room temperature for 1 h and rinsed in 1× PBS for three times (2 min each). After another 1-h incubation with the avidin–biotin peroxidase complex (1:100, Vectastain Elite ABC Kit; Vector Laboratories, CA, USA) and repeated washing steps with 1× PBS, visualization was performed with the chromagen 3,3′-diaminobenzidine (DAB, Dako, Carpinteria, CA, USA). The slides were counterstained with hematoxylin and coverslipped with PerMount. For positive controls, sections of human BC tissue previously found to be TRPS-1, ERα, Her2, and GATA3 positive were used. Isotype IgG and omission of the primary antibody were used as negative controls for staining. Positive cells showed a brownish color in nuclei for TRPS-1, ERα, and GATA3, uniform intense membrane staining for HER2, while negative controls and unstained cells were blue. IHC assays to determine PR status used standard procedures on 4-µm sections of paraffin-embedded tissue specimens stained with the monoclonal antibodies 1A6 (Novacastra Laboratories Ltd., Burlingame, CA, USA) while the case was diagnosed after biopsy.
Quantitative Analysis of IHC Staining
Measurement of TRPS-1, ERα, and GATA3 protein expression in the 152 stage II/III invasive BC samples was performed using computerized image analysis with a SAMBA 4000 Cell Image Analysis System (SAMBA Technologies, Meylan, France) in a blinded fashion without knowledge of the tissue source, diagnosis, or patient outcome measurements. The SAMBA 4000 system is a PC-based articulated system consisting of a Nikon E600 microscope with 10×, 20×, and 40× objectives, a Sony 990MD 3-chip CCD camera, and a Dell computer (Dell Computer Corporation, Austin, TX, USA). Analysis was performed after calibration of the light intensity, and the camera white background was balanced to steady the operating system. In order to insure that the daily variations were minimized to a range of ±1%, a protocol with consistent threshold and color bar was set up and a 20× objective was used to catch the images. Background subtraction was automatically performed on every tissue after storing an empty field of the slide. The SAMBA software (Immuno-Analysis Version 5.02) allows the operator to put an appropriate threshold value for the best differentiation between tissue and background after estimating several fields on the control slide. Then, the threshold for DAB-positive hues (brown) was taken by estimating several fields on positive and negative control slides to separate between blue- (hematoxylin counterstaining) and brown-stained areas optimally. Quantitative computerized image analysis data obtained from nine random fields containing invasive ductal carcinoma components of each section was averaged for each sample. Other nontumor components, including blood vessels, lymphocytes, fibrous connective tissue, ductal carcinoma in situ, and adjacent normal ducts within the fields, were removed in the analysis. In some cases, normal ducts were included in the analysis, while these other areas were excluded. The parameters measured by the imaging system were the labeling index (LI; area of labeled portions/total area of the structures), the mean optical density (MOD; mean labeling concentration), and the quick score (QS), where QS = LI×MOD/100. These parameters were correlated with clinical outcome data.
ERα, PR, and HER2 Status Determination
Nuclear staining >10% was considered a positive result for ERα (ER) and PR. In addition, IHC staining for ER was scored using a semiquantitative scoring system called the Allred score [11, 12, 22] by two pathologists. HER2 was scored using the HercepTest scoring system according to ASCO/CAP guidelines [3, 27] as 0 (no staining of HER2), 1+ (weak staining in any amount of tumor cells), 2+ (at least 10% complete weak membrane staining or less than 30% complete strong membrane staining of tumor cells), and 3+ (uniform strong membrane staining detected in more than 30% of tumor cells). Cases with 3+ staining were considered as having HER2 gene amplification by two pathologists. These were designated as HER2+ in the data analysis in this paper.
Statistical Analyses
Associations between TRPS-1 QS, LI, and MOD of staining with patient and disease characteristics were tested using the Kruskal–Wallis test, Spearman’s correlation coefficient, and Fisher’s exact test as appropriate. Time to event analysis was conducted using the Kaplan–Meier method, and overall survival was calculated from the date of surgery to the date of death from any cause or to the last follow-up date. Cox proportional hazards analysis was used to determine the hazard of death using a TRPS-1 QS and other tumor characteristics in a univariate model. TRPS-1 variables were investigated both as continuous variables and as a categorical variable. All p values were two-tailed and considered significant at alpha <0.05. Analyses were conducted using SAS® for Windows (release 9.1, SAS Institute, Cary, NC, USA) and SPSS® (version 17.0; SPSS Inc., Chicago, IL, USA).
Results
TRPS-1 Expression in Cancerous and Noncancerous Areas in the Stage II/III BC Cases
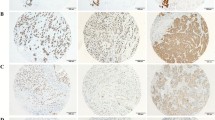
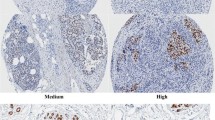
TRPS-1 protein was found to be heterogeneously expressed in the nuclei of the breast cancer cases. Figure 1 shows examples of representative stained tissues from different patients showing the wide range of TRPS-1 QS found in the 152 samples studied. We analyzed different histological regions of ten tumor specimens to determine whether any differences between TRPS-1 expressions exist between cancerous and noncancerous ductal regions. We first analyzed normal ducts in the BC samples and found that they expressed TRPS-1 at a low level. No staining of TRPS-1 was found in myoepithelial cells (Fig. 2a) and stromal tissue. When the levels of TRPS-1 were determined using QS, we found that TRPS-1 was markedly higher in DCIS and invasive ductal carcinoma (IDC) than in adjacent normal ducts or areas of usual ductal hyperplasia (UDH) that may or not be related to the BC (p < 0.05 in each case; Fig. 2b). UDH did not exhibit a statistically significant difference in TRPS-1 QS from normal ducts. These results indicate that TRPS-1 is specifically expressed in luminal epithelial cells, and its expression is significantly increased in cancers of luminal epithelial origin.
Quantitative IHC analysis of TRPS-1 levels in normal ducts and cancerous ducts. a Staining for TRPS-1 in normal ducts (left) and DCIS (right) showing that it is restricted to luminal epithelial cells and not expressed in myoepithelial cells (red arrows). Upper photo is at ×100, and lower photo is at ×200. b TRPS-1 expression in normal ductal epithelial cells, DCIS, and invasive ductal carcinoma as well as areas of usual ductal hyperplasia (UDH) within one representative BC sample from the stage II/III sample set. Tissue samples were processed and stained for TRPS-1 and levels measured using the SAMBA 4000 image analysis system, as described in the “Materials and Methods” section. Magnification used to generate the images was ×100 (upper image) and ×200 (lower image). Note that UDH is shown as an independent entity not related to DCIS or IDC in the progression of BC from normal ducts. The average TRPS-1 QS for IDC and DCIS were significantly higher than for either normal ducts or hyperplasia (p < 0.05), while no significant difference was found between normal ducts and hyperplasia (p > 0.05). c Summary of the TRPS-1 QS measurements in the indicated histological areas from ten patient samples showing the increased expression in DCIS and IDC versus normal ducts and hyperplasia.
TRPS-1 Expression in Association with Other BC Markers
We first correlated TRPS-1 expression (mean QS) using Spearman correlation coefficients with age and the extent of ER and GATA-3 expression (Table 1). TRPS-1, ER, and GATA-3 were all analyzed as continuous variables using their QS values across the sample set. In addition, ER expression relative to TRPS-1 was also analyzed using the Allred score for ER across the sample set. For TRPS-1 staining, 152 samples were able to be evaluated (these sections cut first), while for ER and GATA-3 status, 148 tumor blocks were able to evaluated after sectioning. As shown in Table 1, using TRPS-1 QS was significantly correlated to ER QS (r = 0.24, p = 0.004), ER Allred scores (r = 0.21, p = 0.011), and GATA-3 QS (r = 0.49, p < 0.0001), while not being significantly correlated with age. Using the Kruskal–Wallis test, we further analyzed the association of TRPS-1 QS (median QS across the sample set) with a number of patient clinical parameters (e.g., tumor histology, stage and grade, and lymph node status) in addition to age, ER, PR, GATA-3, and HER2 status that are also prognostic markers in BC (Table 2). ER+ versus ER− status in the sample set was established using a threshold of QS > 5, a LI > 10%, or LI > 5% and an Allred score of greater than 4 [11, 12, 22]. Higher TRPS-1 QS scores were significantly associated with ER+ versus ER− status (p = 0.023 for ER QS ≥ 5 and p = 0.028 for ≥ 4 Allred score, respectively), positive PR status (p = 0.009), and positive HER2 amplification (p = 0.026) status. Interestingly, higher TRPS-1 QS had a trend (p = 0.079) toward being associated with positive ER status determined using LI (≥10% stained cells being ER+), but this was not statistically significant. A similar result was found when a ≥5% LI was used to establish ER+ status (Table 2). Higher TRPS-1 QS was also highly significantly associated with positive GATA-3 status (p < 0.0001; ≥10% LI cutoff for GATA-3+). No significant association was detected between TRPS-1 QS and age category, tumor histology, nodal status, T stage, disease stage, and nuclear grade (Table 2). Interestingly, higher TRPS-1 QS was associated with lymphovascular invasion (p = 0.007; Table 2).
The positive association of TRPS-1 with HER2 amplification (HER2+) prompted us to further investigate how different subsets of ER+ and ER− cancers expressed TRPS-1 relative to HER2 status. Both TRPS-1 status (as QS) and ER status (LI and QS) were determined using our qIHC protocol, as described above. This analysis showed that TRPS-1 had a significantly higher expression in ER+HER2+ BC than ER+HER2− (p < 0.05) using both ER evaluation methods (Supplementary Table 1 online). In ER− tumors, TRPS-1 QS had a tendency to be higher in ER−HER2+ subset, but this was not statistically significant.
TRPS-1 Staining in Male BC and Other Major Types of Cancer
We were interested to determine whether or not TRPS-1 was also expressed in BC from males and not restricted to female BC. Eleven samples of previously diagnosed stage II/III male IDC were analyzed for TRPS-1 using qIHC. TRPS-1 was expressed in the male BC cases with an average LI, MOD, and QS of 68.2%, 28.5, and 20.5, respectively. We also performed IHC and qIHC analysis of TMA from other forms of cancer that can occur in females (lung, ovarian, colorectal, and endometrial cancer) to determine whether TRPS-1 is expressed in other forms of cancer. A more detailed description of these TMA samples and histological subtypes are provided in Supplementary Table 2 online. As shown in Fig. 3, TRPS-1 was most prevalent in BC with 92.2% of tumors scoring positive. Staining for TRPS-1 in colorectal cancer was completely negative, while lung, ovarian, and endometrial cancers had 17.4%, 20.3%, and 12.3% of specimens scoring positive in the TMA samples, respectively. Of the positive tumors in these samples, the level of TRPS-1 expression, as determined by QS analysis, was also markedly higher in BC than in other forms of cancer (Table 3).
Comparison of TRPS-1 expression levels by qIHC in breast, lung, colorectal, endometrial, and ovarian cancer in TMA shows that TRPS-1 is the most prevalent and has the highest QS (LI and MOD) in breast cancer. Tissue microarrays of the indicated cancer types were stained with the optimized IHC staining protocol with the 8D11 mAb followed by measurement of LI and MOD using the SAMBA 4000 System. Photomicrographs shown are at ×100. Description of the stage and subtype of the different cancer types in the different TMAs is provided in Supplementary Table 2 online.
TRPS-1 Expression and Overall Survival in the Stage II/III BC Patient Cohort
The differential expression of TRPS-1 via qIHC across the patient sample set led us to further analyze the possible prognostic relevance of TRPS-1 in terms of overall survival relative to TRPS-1 QS. The maximum duration of follow-up for the 152 stage II/III BC cases was 18.15 years. During this period of time, the overall survival was 68.8%. Cox proportional hazards analysis was performed using a univariate model assessing the predictive value of TRPS-1 QS relative to other patient and tumor characteristics (Table 4). When analyzed as a continuous variable, neither TRPS-1, ER, nor GATA-3 QS was significantly associated with better or worse survival in terms of hazard of death (HR; data not shown). However, when analyzed as a categorical variable, TRPS-1 QS <4 was significantly associated with worse survival with an HR ratio of 2.0 (95% confidence interval (CI) 1.12 to 3.55, p = 0.019; Table 4). We also looked at other TRPS-1 QS categories (cutoffs of less than 4 or higher than 4) but found that these were not predictive (data not shown). PR-negative status and T stage (T3 or T4) were also found to be predictive of worse survival (HR = 1.94, p = 0.031 for PR; HR = 2.29, p = 0.008 for T stage, respectively). The predictive value of ER was analyzed using our QS and LI analysis, as well as Allred score. Using our QS determination for ER, significantly worse survival (HR = 2.08, p = 0.017) was found for patients with primary tumors having QS <5 for ER versus QS ≥ 5 (Table 4). A low ER Allred Score (<4) had an insignificant trend (HR = 1.792, p = 0.058) toward worse survival, while, interestingly, when either a ≥10% LI cutoff or a ≥5% LI cutoff was used for gauging ER status, this was not predictive in a statistically significant manner (Table 4). In addition, GATA-3 status was also not predictive in this regard (Table 4). Kaplan–Meier survival analysis also showed a significantly improved survival of patients having a TRPS-1 QS ≥4 versus <4 (p = 0.0165; Fig. 4). Other variables investigated that did not achieve statistical significance in predicting overall survival included patient age, tumor stage, grade, tumor histology, lymphovascular invasion, and HER2 status.
Kaplan–Meier analysis of TRPS-1 QS and overall survival in the 152 stage II/III breast cancer sample set. TRPS-1 was stained and analyzed using the SAMBA 4000 image analysis system as described in the “Materials and Methods.” Survival probability (in years from primary surgery) was evaluated for TRPS-1 QS at >4 versus ≤4. Inset shows the p value (p = 0.0165) calculated using log rank analysis.
Discussion
Previously, we reported that the TRPS-1 gene was expressed in approximately 90% of BC samples and was one of the most prevalent genes in BC after a differential gene expression screen using RNA from over 60 primary surgical tumor specimens in comparison to over 200 normal tissue RNA samples [20]. TRPS-1 is a new member of the GATA family of transcriptional regulators having a demonstrated transcriptional repressor function by inhibiting GATA-induced gene expression [6, 7, 25]. Recently, GATA-3 has been found to be a key mediator of luminal ductal cell differentiation and is an important mediator in ER signaling driving ER+ BC growth and survival [1, 2, 9, 18, 24]. For example, GATA-3 and ER have been found to reciprocally regulate the expression of the other [10, 13]. We recently have found that induction of TRPS-1 in BC cells (MCF-7) inhibits GATA-3-induced gene expression using a luciferase reporter construct and also inhibits the expression of ER- and GATA-3-induced genes such as CYP19 and MYB (Bao et al., unpublished observations). Thus, given this transcriptional repressor role of TRPS-1, we decided to further investigate the nature of TRPS-1 expression in primary human BC samples and its association with major breast tumor histological and patient clinical characteristics. We also performed an initial evaluation of the possible prognostic role of TRPS-1. We evaluated these parameters in a set of randomly chosen primary surgical specimens from 152 stage II/III BC patients who had not received neoadjuvant chemotherapy.
TRPS-1 was found only in ductal epithelium and not in myoepithelial cells, indicating its specific association with luminal cells and cancers arising from these cells. In addition, we found high TRPS-1 expression in all 11 samples of male invasive ductal carcinoma studied. We also stained for TRPS-1 in other common cancers among women by IHC and found that TRPS-1 was most prevalent in BC, with lower levels of expression in lung, ovarian, and endometrial cancer and no expression found in colorectal cancer. Thus, TRPS-1 is most prevalent in BC in women and seems to be a specific luminal ductal epithelial cell marker in the breast.
Due to the high percentage of BC scoring positive for TRPS-1 [20], we could not simply quantify TRPS-1 expression after IHC using subjective cutoffs of the percentage of positively staining tumor cells in each sample. Rather, we needed to develop a highly precise qIHC method to measure TRPS-1 expression. This qIHC approach found that TRPS-1 was significantly expressed in normal ductal epithelium but was overexpressed in both DCIS and invasive ductal carcinoma lesions (Fig. 2). Of note was that, overall, TRPS-1 was not overexpressed in areas of noncancerous hyperplasia within the patient samples and was found at similar levels in these areas as in normal ducts. Thus, TRPS-1 overexpression seems to be induced during BC initiation. The reason for this increased expression in not known but may be due to enhanced transcriptional activity in the chromosome 8q23-24 region where TRPS-1 is located; 8q23-24 is a common area of increased transcriptional activity and amplification in BC [21] containing other genes previously demonstrated to be overexpressed in BC such as c-myc [21]. Further analysis of TRPS-1 expression versus other major BC markers found that TRPS-1 QS was highly positively associated with ER, PR, and GATA-3 expression status, with higher QS associated with ER+, PR+, and GATA-3+ BC.
TRPS-1 QS was also higher in ER+HER2-amplified (HER2+) BC, corroborating our initial results [20] on a small set of 30 cases, suggesting that it is expressed in all forms of ductal BC and has preferential expression in ER+ BC. Closer analysis of different BC subsets based on ER and HER2 amplification (HER2+ or HER2−) status revealed that TRPS-1 was expressed more highly in ER+HER2+ versus ER+HER2− BC. In the more limited number of ER− BC samples, TRPS-1 expression showed a tendency towards higher expression in the ER−HER2+ versus the ER−HER2− subset, further indicating its association with a ductal epithelial phenotype rather than a basal phenotype. Interestingly, we did find TRPS-1 expression in some basal phenotype (triple-negative) tumors (ER/PR− and HER2−) that stained positive for CK5/6 and EGF receptor (Chen et al., unpublished observations). If TRPS-1 turns out to be a specific marker for ductal epithelium, then it is tempting to speculate that these TRPS-1 “basal-like” tumors may actually be of ductal rather than basal cell origin and have a different disease course than actual basal tumors. This is currently being investigated in a larger stage I–III sample set.
Although this sample set was not optimal in terms of sample size, we did perform a survival analysis based on TRPS-1 QS analysis and found some interesting results worth following up on. Interestingly, when evaluated as continuous QS variables, neither TRPS-1 nor ER and GATA-3 levels were statistically associated with differences in survival. This was surprising considering the well-known positive prognostic significance of ER in small as well as larger BC sample sets [2]. This underscores how quantitative IHC can lead to different results versus traditional subjective cutoffs categorizing expression as simply positive versus negative. However, when used as categorical variables, TRPS-1 and ER were found to be predictive, with QS above a certain cutoff in each case associated with improved overall survival. Both univariate Cox proportional hazards analysis and Kaplan–Meier analysis found that a TRPS-1 QS as a categorical variable (QS ≥ 4) was significantly associated with better survival, with a QS <4 predicting a twofold increased probability of death within the 18.6 study time period. This HR was similar to that predicted with ER-negative tumor status, as found here and in previous studies [2]. Although this analysis needs to be repeated with a larger sample set (currently underway), it does indicate that TRPS-1 can be a new prognostic marker in BC that should be further evaluated in future studies not only in ER+ BC but also as a possible prognostic indicator in ER− BC, where due to the lack of ER and all of its associated positive effects, TRPS-1 expression may emerge to be a stronger indicator of better survival. In this regard, although TRPS-1 is preferentially associated with ER, PR, and GATA-3 expression, we have found expression in a significant number of ER− BC, especially those with HER2 amplification.
Currently, the molecular biology surrounding the function of the TRPS-1 gene in BC cells is under investigation. We have cloned and sequenced the full-length TRPS-1 gene from human BC cell lines and identified it as a 1,294-amino-acid transcriptional regulator [20]. Sequencing of the TRPS-1 gene amplified from a number of BC cell lines (e.g., MCF7, SKBR3) has found no mutations in the gene, indicating that a wild-type form of the protein is expressed in BC cells (Bao et al., unpublished observations). Our initial analysis using TRPS-1 lentiviral transduction of TRPS-1− BC cells suggests that it inhibits GATA-3 transcription factor function as predicted and promotes apoptosis, as we have not been able to generate stable TRPS-1-overexpressing clones from TRPS-1− BC cells (Bao et al., unpublished observations). Thus, BC cells not conditioned to express high levels of TRPS-1 may not tolerate induction of higher TRPS-1 levels. These results are in agreement with the previously demonstrated function of TRPS-1 in androgen-dependent prostate cancer cells, where it has been shown to facilitate apoptosis and inhibit GATA-dependent expression of PSA [25]. Interestingly, the work in prostate cancer suggested that TRPS-1 protein expression was downregulated by androgen receptor signaling, as androgen withdrawal induced TRPS-1 expression and apoptosis [5, 6]. Thus, the relationship between ER signaling (versus simply ER expression) and TRPS-1 expression and function in BC needs to be studied. A possible parallel scenario in ER+ BC cells is that TRPS-1 expression may increase to high levels and be part of an apoptotic program when ER signaling is suppressed.
In summary, TRPS-1, a new member of the GATA transcription factor family, is highly prevalent in BC with some expression in other forms of cancer but at significantly lower levels. TRPS-1 expression using qIHC was significantly correlated with ER, PR, GATA-3 as well as HER2. Patients who had a level of TRPS-1 above a certain threshold had markedly improved overall survival. Our results suggest that TRPS-1 may be a tumor suppressor gene worthy of further investigation as a possible prognostic marker in larger patient sample sets, where additional parameters such as relapse-free survival and time to relapse during antihormone therapy can be evaluated. The need for further studies on the prognostic value of TRPS-1 is also supported by its molecular function as transcriptional repressor of GATA-dependent genes critical in hormone signaling. These studies should also analyze TRPS-1 as prognostic markers in ER+ and ER− BC subtypes, as well as the effect of TRPS-1 on the estrogen signaling pathway in BC cells. Lastly, our results point to the potential power of using a quantitative approach to IHC to measure the prognostic value of critical genes in BC and other cancer types. Due to the high prevalence of TRPS-1 in BC at different stages and in normal ducts (90% or higher), we needed to develop a qIHC methodology combining LI with intensity of expression (MOD) in a combined variable (QS) that could be used to analyze differences between patient samples, disease stages, and between diseased versus normal tissue. Indeed, using this approach, we found significant variation in LI and MOD across the stage II/III BC sample set studied here that resulted in a wide distribution of QS. This significant variation would have been masked by traditional methods of IHC scoring using subjective cutoffs (e.g., 10% or higher staining being scored as positive). Overall, this qIHC approach may be useful for other critical BC-related genes that, although having a high prevalence, may exhibit differential levels of protein expression intensities and intratumor heterogeneity that can be combined in an objective quantitative variable that may be much more biologically informative that current approaches using subjective staining cutoffs.
Abbreviations
- BC:
-
Breast cancer
- DSS:
-
Disease-specific survival
- ER:
-
Estrogen receptor alpha
- IHC:
-
Immunohistochemistry
- LI:
-
Labeling index
- mAb:
-
Monoclonal antibody
- MOD:
-
Mean optical density
- OS:
-
Overall survival
- PSA:
-
Prostate-specific antigen
- AR:
-
Androgen receptor
- PR:
-
Progesterone receptor
- qIHC:
-
Quantitative immunohistochemistry
- QS:
-
Quick score
- RFS:
-
Relapse-free survival
- TRPS-1:
-
Trichorhinophalangeal syndrome 1
References
Asselin-Labat ML, Sutherland KD, Barker H et al (2007) GATA-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9:201–209
Badve S, Nakshatri H (2009) Oestrogen-receptor-positive breast cancer: towards bridging histopathological and molecular classifications. J Clin Pathol 62:6–12
Birner P, Oberhuber G, Stani J et al (2001) Evaluation of the United States food and drug administration-approved scoring and test system HER-2 protein expression in breast cancer. Clin Cancer Res 7:1669–1675
Black M, Speer F (1982) Nuclear structure in cancer tissue. Surg Gynecol Obstet 105:97–102
Chang GTG, Gamble SC, Jhamai M et al (2007) Proteomic analysis of proteins regulated by TRPS-1 transcription factor in CU145 prostate cancer cells. Biochim Biophys Acta 1774:575–582
Chang GTG, Jhamai M, van Weerden WM, Jenster G, Brinkmann AO (2004) The TRPS1 transcription factor: androgenic regulation in prostate cancer and high expression in breast cancer. Endocr Relat Cancer 11:815–822
Chang GTG, van den Bemd GJCM, Jhamai M, Brinkmann AO (2002) Structure and function of GC79/TRPS1, a novel androgen-repressible apoptosis gene. Apoptosis 7:13–21
Ciocca V, Daskalakis C, Ciocca RM et al (2009) The significance of GATA3 expression in breast cancer: a 10-year follow-up study. Human Pathol 40:489–495
Ciocca V, Daskalakis C, Ciocca RM et al (2009) The significance of GATA3 expression in breast cancer: a 10-year follow-up study. Hum Pathol 40:489–95
Fang SH, Chen YZ, Weigel RJ (2008) GATA-3 as a marker of hormone response in breast cancer. J Surg Res 148:1–6
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481
Harvey JM et al (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–81
Hoch RV, Thompson DA, Baker RJ et al (1999) GATA-3 is expressed in association with estrogen receptor in breast cancer. Int J Cancer 84:122
Kaiser FJ, Brega P, Raff ML et al (2003) Novel missense mutations in the TRPS1 transcription factor define the nuclear localization signal. Eur J Hum Genet 12:121–126
Lüdecke HJ, Schaper J, Meinecke P et al (2001) Genotypic and phenotypic spectrum in trichorhinophalangeal syndrome types I and III. Am J Hum Genet 68:81–91
Malik TH, von Stechow D, Bronson RT, Shivdasani RA (2002) Deletion of the GATA domain of TRPS1 causes an absence of facial hair and provides new insights into the bone disorder in inherited tricho-rhino-phalangeal syndromes. Mol Cell Biol 22:8592–8600
Momeni P, Glöckner G, Schmidt O et al (2000) Mutations in a new gene, encoding a zinc-finger protein, cause tricho-rhino-phalangeal syndrome type I. Nat Genet 24:71–74
Parikh P, Palazzo JP, Rose LJ et al (2005) GATA-3 expression as a predictor of hormone response in breast cancer. J Am Coll Surg 200:705–10
Perdomo J, Crossley M (2002) The Ikaros family protein EOS associates with C-terminal-binding protein corepressors. Eur J Biochem 269:5885–5892
Radvanyi L, Singh-Sandhu D, Gallichan S et al (2005) The gene associated with trichorhinophalangeal syndrome in humans is over-expressed in breast cancer. Proc Natl Acad Sci U S A 102:11005–11010
Savinainen K, Linja M, Saramäki O et al (2004) Expression and copy number analysis of TRPS1, EIF3S3 and MYC genes in breast and prostate cancer. Br J Can 90:1041–1046
Shousha S (2008) Oestrogen receptor status of breast carcinoma: Allred/H score conversion table. Histopathology 53:346–347
Tlsty TD (2007) Luminal cells GATA have it. Nat Cell Biol 9:135–136
Tong Q, Hotamisligil GS (2007) Developmental biology: cell fate in the mammary gland. Nature 445:724–6
van den Bemd G-JCM, Jhamai M, Brinkmann AO, Chang GTG (2003) The atypical GATA protein TRPS1 represses androgen-induced prostate-specific antigen expression in LNCaP prostate cancer cells. Biochem Biophys Res Com 312:578–584
WHO (1982) The World Health Organization histological typing of breast tumors-second edition. Am J Clin Pathol 78:806–816
Wolff AC, Hammond MEH, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Acknowledgements
This work was supported by a grant from the Cancer Vaccine Program of Sanofi Pasteur Canada to MD Anderson Cancer Center (LS2006-00015466AG). We thank Drs. Michael Gilrease and Erika Resetkova (Dept. of Pathology, M.D. Anderson Cancer Center) for critically reading and commenting on the manuscript.
Competing Interests
LGR receives research grant support from Sanofi Pasteur Canada. NL was an employee of Sanofi Pasteur. A provisional patent application on the quantitative IHC method for TRPS-1 staining has been filed with the US Patent Office.
Authors Contributions
LGR conceived of the project. JQC, LX, HZZ, and SW performed the experiments. JQC, JL, HZZ, RK, NL, JLM, and LGR helped plan experiments. JL, JQC, HZZ, CW, and LGR interpreted data and wrote the paper. YW, XYS, AS, GH, and MB secured patient samples and reviewed the manuscript. GB and NL critically reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jie Qing Chen and Jennifer Litton contributed equally to this study.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Association of TRPS-1 QS with subtypes of BC based on the combination of ER and HER2 amplification (HER2+) using ER status determined using LI or QS cutoffs established using qIHC. (DOC 37 kb)
Supplementary Table 2
Histologic type of lung, ovarian, endometrial, and colorectal cancer samples in the different TMAs used to analyze TRPS-1 expression. (DOC 36 kb)
Rights and permissions
About this article
Cite this article
Chen, J.Q., Litton, J., Xiao, L. et al. Quantitative Immunohistochemical Analysis and Prognostic Significance of TRPS-1, a New GATA Transcription Factor Family Member, in Breast Cancer. HORM CANC 1, 21–33 (2010). https://doi.org/10.1007/s12672-010-0008-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-010-0008-8