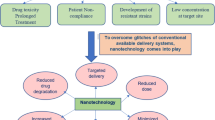
Abstract
Nanoscience is emerging as a new era of technology advancement which has promoted our ability to understand scientific developments at nanoscale. The nanoparticle-based drug delivery systems have been implicated in the treatment of a variety of diseases especially the ones caused by intracellular pathogens. Therapeutic failures and antibiotic resistance developed by the notorious pathogenic bacteria like Mycobacterium tuberculosis have prompted researchers to develop novel ways to counter drug resistance, to shorten the treatment duration, and more importantly to reduce drug interactions with antiretroviral therapies. The pharmaceutical technologists of today are focusing more on improving the effectiveness of the drug by specifically targeting the sources and reservoirs of infections. The nanotechnology has the potential to develop more effective and compliant medicines. Current review discusses about various challenges faced in the development of effective nano-based tuberculosis therapies and overviews various state-of-art technologies being developed in-terms of nano-based drug delivery systems for encapsulation and sustained release of antituberculosis drugs.

Similar content being viewed by others
References
Bawa, R., Bawa, S. R., Maebius, S. B., Flynn, T., Wei, C. (2005). Protecting new ideas and inventions in nanomedicines with patents. Nanomedicine: Nanotechnology, Biology and Medicine, 1, 150–158.
Butler, J. C., Hofman, J., Cetron, M. S., Elliot, J. A., Arancibia, F., et al. (1996). The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the centres for disease control and prevention’s Pneumococcal Sentinal Surveillance System. Journal of Infectious Diseases, 174, 986–993.
Kunin, C. M. (1993). Resistance to antimicrobial drugs—a world-wide calamity. Annals of International Medicine, 118, 557–561.
Petrini, B., & Hoffner, S. (1999). Drug resistant and multidrug resistant tubercle bacilli. International Journal of Antimicrobial Agents, 13, 93–97.
Dye, C., Sheele, S., Dolin, P., Pathania, V., Raviglione, M. C. (1999). Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. Journal of the American Medical Association, 282, 677–686.
Wright, A. (2006). Emergence of Mycobacteria tuberculosis with extensive resistance to second line drugs worldwide. Journal of the American Medical Association, 295, 2349–2351.
Gonda, I., Schuster, J. A., Rubsamen, R. M., Lloyd, P., Cipolla, D., et al. (1998). Inhalation delivery systems with compliance and disease management capabilities. Journal of Controlled Release, 53, 269–274.
Swai, H., Semete, B., Kalombo, L., Chellule, P., Kisich, K., Sievers, B. (2009). Nanomedicine for respiratory diseases. Wires nanomed nanobiotechnol, 1, 255–263.
Prabakaran, D., Singh, P., Jaganathan, K. S., Vyas, S. P. (2004). Osmotically regulated asymmetric capsular systems for simultaneous sustained delivery of anti-tubercular drugs. Journal of Controlled Release, 95(2), 239–248.
Constantinides, P. P., Chaubal, M. V., Shorr, R. (2008). Advances in lipid nano-dispersions for parenteral drug delivery and targeting. Advanced Drug Delivery Reviews, 60, 757–767.
Seki, J., et al. (2004). A nanometer lipid emulsion, lipid nano-sphere (LNS) as a parenteral drug carrier for drug targeting. International Journal of Pharmacology, 273(1–2), 75–83.
Singh, S. K., et al. (2008). Phosphoethanolamine-complexed C-reactive protein: a pharmacological-like macromolecule that binds to native low-density lipoprotein in human serum. Clinical Chim Acta, 394(1–2), 94–98.
Ahmed, M., Ramadan, W., Rambhu, D., Shakeel, F. (2008). Potential of nano-emulsions for intravenous delivery. Pharmazie, 63, 806–811.
Gelperina, S., Kisich, K., Iseman, M. D., Heifets, L. (2005). The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. American Journal of Respiratory and Critical Care Medicine, 172, 1487–1490.
Sosnik, A., Carcaboso, A. M., Glisoni, R. J., Moretton, M. A., Chiappetta, D. A. (2010). New old challenges in tuberculosis: potentially effective nanotechnologies in drug delivery. Advanced Drug Delivery Reviews, 62, 547–559.
Rabinow, B. E. (2004). Nanosuspensions in drug delivery. Nature Reviews. Drug Discovery, 3, 785–796.
Williart, J. F., & Descamps, M. (2008). Solid state amorphization of pharmaceuticals. Molecular Pharmacology, 5, 905–920.
Kesisoglou, F., Panmai, S., Wu, Y. (2007). Nano-sizing oral formulation development and biopharmaceutical evaluation. Advanced Drug Delivery Reviews, 59, 631–644.
Peters, K., et al. (2000). Preparation of a clofazimine nano-suspension for intravenous use and evaluation of its therapeutic efficacy in murine Mycobacterium avium infection. Journal of Antimicrobial Chemotherapy, 45, 77–83.
Reverchon, E., DeMarco, I., DellaPorta, G. (2002). Rifampicin microparticle production by supercritical antisolvent precipitation. International Journal of Pharmacology, 243, 83–91.
Reverchon, E., & DellaPorta, G. (2003). Micronization of antibiotics by supercritical assisted atomization. Journal of Supercritical Fluids, 26, 243–252.
Sahin, N. O. (2007). Niosomes as nanocarrier systems. In M. R. Moza (Ed.), Nanomaterials and nanosystems for biomedical applications (pp. 67–81). the Netherlands: Springer.
Jain, C. P., & Vyas, S. P. (1995). Preparation and characterization of niosomes containing rifampicin for lung targeting. Journal of Microencapsulation, 12, 401–407.
Jain, C. P., Vyas, S. P., Dixit, V. K. (2006). Niosomal system for delivery of rifampicin to lymphatics. Indian Journal of Pharmacology, 68, 575–578.
Mullaicharam, A. R., & Murthy, R. S. R. (2004). Lung accumulation of niosome entrapped rifampicin following intravenous and intratracheal administration in the rat. Journal of Drug Delivery Science and Technology, 14, 99–104.
Soppimath, K. S., Aminabhavi, T. M., Kulkarni, A. R., Rudzinski, W. E. (2001). Biodegradable polymeric nanoparticles as drug delivery devices. Journal of Controlled Release, 70, 1–20.
Sharma, A. K., & Khuller, G. K. (2001). DNA vaccines—future strategies and relevance against intracellular pathogens. Immunology & Cell Biology, 79(6), 537–546.
Delie, F., & Blanco-Prieto, M. J. (2005). Polymeric particulates to improve oral bioavailability of peptide drugs. Molecules, 10, 65–80.
Sharma, A. K., Verma, I., Tewari, R., Khuller, G. K. (1999). Adjuvant modulation of T-cell reactivity to 30 kDa secretory protein of Mycobacterium tuberculosis H37Rv and its protective efficacy against experimental tuberculosis. Journal of Medical Microbiology, 48, 757–763.
Pandey, R., Sharma, A., Zahoor, A., Sharma, S., Khuller, G. K., Prasad, B. (2003). Poly(DL-lactide-co-glycolide nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. Journal of Antimicrobial Chemotherapy, 52, 981–986.
Semete B., et al. (2010) In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine: Nanotechnology, Biology and Medicine, 6, 662–671.
Croy, S. R., & Kwon, G. S. (2006). Polymeric micelles for drug-delivery. Current Pharmaceutical Design, 12, 4669–4684.
Gao, P., Nie, X., Zou, M., Shi, Y., Cheng, G. (2011). Recent advances in materials for extended-release antibiotic delivery system. Journal of Antibiotics (Tokyo). doi:10.1038/ja.2011.58.
Kisich, K. O., et al. (2007). Encapsulation of moxifloxacin within poly(butyl cyanoacrylate) nanoparticles enhances efficacy against intracellular Mycobacterium tuberculosis. International Journal of Pharmacology, 345, 154–162.
Shipulo, E., et al. (2008). Development of nanosomal formulation of moxifloxacin based on poly(butyl-2-cyanoacrylate). Pharmaceutical Chemistry Journal, 42, 145–149.
Dutt, M., & Khuller, G. K. (2001). Chemotherapy of Mycobacterium tuberculosis infections in mice with a combination of isoniazid and rifampicin entrapped in poly (DL-lactide-co-glycolide) microparticles. Journal of Antimicrobial Chemotherapy, 47(6), 829–835.
Ain, Q., Sharma, S., Garg, S. K., Khuller, G. K. (2002). Role of poly [DL-lactide-co-glycolide] in development of a sustained oral delivery system for antitubercular drug(s). International Journal of Pharmacology, 239(1–2), 37–46.
Gangadharam, P. R., Geeta, N., Hsu, Y. Y., Wise, D. L. (1999). Chemotherapy of tuberculosis in mice using single implants of isoniazid and pyrazinamide. International Journal of Tuberculosis and Lung Disease, 3(6), 515–520.
Kailasam, S., Daneluzzi, D., Gangadharam, P. R. (1994). Maintenance of therapeutically active levels of isoniazid for prolonged periods in rabbits after a single implant of biodegradable polymer. Tubercle and Lung Disease, 75(5), 361–365.
Vyas, S. P., Kannan, M. E., Jain, S., Mishra, V., Singh, P. (2004). Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages. International Journal of Pharmacology, 269(1), 37–49.
Wissing, S. A., Kayser, O., Muller, R. H. (2004). Solid lipid nanoparticles for parenteral drug delivery. Advanced Drug Delivery Reviews, 56(9), 1257–1272.
Pandey, R., & Khuller, G. K. (2005). Antitubercular inhaled therapy: opportunities, progress and challenges. Journal of Antimicrobial Agents and Chemotherapy, 55, 430–435.
Yan, W., Li, M., Gao, H. (2009). Polymeric micelle composed of PLA and chitosan as a drug carrier. Journal of Polymer Research, 16, 11–18.
Silva, M., et al. (2001). Potential tuberculostatic: micelle-forming copolymer poly(ethylene glycol) poly(aspartic acid) prodrug with isoniazid. Arch Pharma Med Chem, 334, 189–193.
Silva, M., et al. (2006). Potential tubercular anti TB agent: micelle forming pyrazinamide prodrug. Arch Pharma Chem, 339, 283–290.
Silva, M., et al. (2007). Preparation of polymeric micelles for use as carriers of tuberculostatic drugs. Tropical Journal of Pharmaceutical Research, 6, 815–824.
Kumar, P. V., Asthana, A., Dutta, T., Jain, N. K. (2006). Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. Journal of Drug Targeting, 14(8), 546–556.
Kumar, P. V., Agashe, H., Dutta, T., Jain, N. K. (2007). PEGylated dendritic architecture for the development of a prolonged drug delivery system for an antitubercular drug. Current Drug Delivery, 4(1), 11–19.
Rao, B. P., Suresh, S., Narendra, C., Balasangameshwer. (2006). Physicochemical characterization of a-cyclodextrin and hydroxyethyl a-cyclodextrin complexes of rifampicin. Ars Pharmaceutica, 47, 37–59.
Klemens, S. P., Cynamon, M. H., Swenson, C. E., Ginsberg, R. S. (1990). Liposome-encapsulated-gentamicin therapy of Mycobacterium avium complex infection in beige mice. Antimicrobial Agents and Chemotherapy, 34(6), 967–970.
Oh, Y. K., Nix, D. E., Straubinger, R. M. (1995). Formulation and efficacy of liposome encapsulated antibiotics for the therapy of intracellular Mycobacterium avium infection. Antimicrobial Agents and Chemotherapy, 39, 2104–2111.
Leitzke, S., et al. (1998). Rationale for and efficacy of prolonged interval treatment using liposome encapsulated amikacin in experimental Mycobacterium avium infection. Antimicrobial Agents and Chemotherapy, 42, 459–461.
Deol, P., Khuller, G. K., Joshi, K. (1997). Therapeutic efficacy of isoniazid and rifampin encapsulated in lung-specific stealth liposomes against Mycobacterium tuberculosis infection induced in mice. Antimicrobial Agents and Chemotherapy, 41, 1211.
Tomalia, D. A. (2005). Birth of new molecular architecture: dendrimers as quantized building blocks for nano-scale synthetic polymer chemistry. Progress in Polymer Science, 30, 294–324.
Evrard, B., et al. (2004). Cyclodextrins as a potential carrier in drug nebulization. Journal of Controlled Release, 96, 403–410.
Antoni, P., et al. (2009). Bifunctional dendrimers: from robust synthesis and accelerated one-pot postfunctionalization strategy to potential applications. Int. Ed. Engl., 48(12), 2126–2130.
McElhanon, J. R., & McGrath, D. V. (2000). Toward chiral polyhydroxylated dendrimers. Preparation and chiroptical properties. Journal of Organic Chemistry, 65(11), 3525–3529.
Liang, C. O., & Fréchet, J. M. J. (2005). Incorporation of functional guest molecules into an internally functionalizable dendrimer through olefin metathesis. Macromolecules, 38(15), 6276–6284.
Hecht, S., & Fréchet, J. M. J. (2001). Dendritic encapsulation of function: applying nature’s site isolation principle from biomimetics to materials science. Angewandte Chemie International Edition, 40(1), 74–91.
Frechet, J. M. J. (1994). Functional polymers and dendrimers: reactivity, molecular architecture, and interfacial energy. Science, 263(5154), 1710–1715.
Kumar, T. R., Dutta, T., Gajbhiye, V., Jain, N. K. (2009). Exploring dendrimer towards dual drug delivery. Journal of Microencapsulation (Pharmaceutics Research Laboratory, Department of Pharmaceutical Sciences, Dr Hari Singh Gour University), 26(4), 287–296.
Dutta, T., & Jain, N. K. (2007). Targeting potential and anti HIV activity of mannosylated fifth generation poly (propyleneimine) dendrimers. Biochimica et Biophysica Acta, 1770, 681–686.
Dutta, T., et al. (2007). Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro. Journal of Drug Targeting, 15(1), 84–96.
Dutta, T., Garg, T., Jain, N. K. (2008). Targeting of efavirenz loaded tuftsin conjugated poly(propyleneimine) dendrimers to HIV infected macrophages in vitro. European Journal of Pharmaceutical Sciences, 34(2–3), 181–189.
Fischer, M., & Vogtle, F. (1999). Dendrimers: from design to application—a progress report. Angewandte Chemie International Edition, 38(7), 884–905.
Fu, H. L., Cheng, S. X., Zhang, X. Z. (2007). Dendrimer/DNA complexes encapsulated in a water soluble polymer and supported on fast degrading star poly(DL-lactide) for localized gene delivery. Journal of Control Release (Key Laboratory of Biomedical Polymers of Ministry of Education, Department of Chemistry, Wuhan University), 124(3), 181–188.
Fu, H. L., Cheng, S. X., Zhang, Z. X., Zhuo, R. X. (2008). Dendrimer/DNA complexes encapsulated functional biodegradable polymer for substrate-mediated gene delivery. The Journal of Gene Medicine (Key Laboratory of Biomedical Polymers of Ministry of Education, Department of Chemistry, Wuhan University, Wuhan, People’s Republic of China), 10(12), 1334–1342.
Sham, J. O., Zhang, Y., Finlay, W. H., Roa, W. H., Lobenberg, R. (2004). Formulation and characterization of spray dried powders containing nano-particles for aerosol delivery to the lung. International Journal of Pharmacology, 269, 457–467.
Sharma, A., Sharma, S., Khuller, G. K. (2004). Lectin functionalized poly(lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. Journal of Antimicrobial Agents and Chemotherapy, 54, 761–766.
Pandey, R., & Khuller, G. K. (2004). Subcutaneous nano-particle based antitubercular chemotherapy in an experimental model. Journal of Antimicrobial Agents and Chemotherapy, 54, 266–268.
Mathuria, J. P. (2009). Nanoparticles in tuberculosis diagnosis, treatment and prevention: a hope for future. Digest Journal of Nanomaterials and Biostructures, 4(2), 309–312.
Xenariou, S., Griesenbach, U., Ferrari, S., Dean, P., Schule, R. K., Cheng, S. H., et al. (2006). Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivo. Gene Therapy, 13, 1545–1552.
Ziardy, A. G., et al. (2003). Transfection of airway epithelium by stable PEGylated poly L-lysine DNA nano-particles in vivo. Molecular Therapy, 8, 936–947.
Konstan, M. W., et al. (2004). Compacted DNA nano-particles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Human Gene Therapy, 15, 1255–1269.
Gwinn, M. R., & Vallyathan, V. (2006). Nanoparticles: health effects—pros and cons. Environmental Health Perspectives, 114(12), 1818–1825.
Oberdorster, G., Oberdorster, E., Oberdorster, J. (2005). Nano-toxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspective, 113, 823–839.
Pandey, R. (2005). Antitubercular inhaled therapy: opportunities, progress and challenges. Journal of Antimicrobial Chemotherapy, 55, 430.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, A.K., Kumar, R., Nishal, B. et al. Nanocarriers as Promising Drug Vehicles for the Management of Tuberculosis. BioNanoSci. 3, 102–111 (2013). https://doi.org/10.1007/s12668-013-0084-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-013-0084-7