Abstract
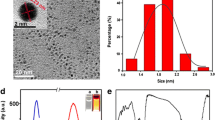
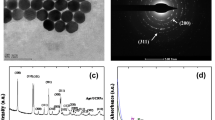
The present study focuses on the construction of optical nanobiosensor to determine toxicological behavior of mitoxantrone (MTX), an anti-tumor drug. For this purpose, gold nanoparticles (AuNPs) were labeled with DNA, respectively combining the physicochemical detector component with the biological analytical component. Fluorescence resonance energy transfer (FRET) phenomenon works as the basic principle for the determination of MTX–DNA interaction by spectrofluorometry. Mitoxantrone intercalates with the DNA and produces MTX-DNA adduct, which restricts protein synthesis and causes excessive production of free radicals in the myocardium that could eventually lead to cardiac arrest. Mapping the adverse reaction of MTX with DNA at molecular level, a conformational change within the nanobiosensor complex was evident that increased the distance between the fluorescent/quencher molecules. The consequent changes in the fluorescence spectrum of the sensor due to FRET modulation by varying concentrations of MTX proved the basis of the tox-screen. Paracetamol, an analgesic agent, was used as controlled drug in this study. Results have demonstrated that the optical nanobiosensor is rapid and sensitive with a detection limit up to 1 μg of MTX interaction, illustrating how it is a feasible technique for surveillance of drug–DNA interaction in molecular toxicology.









Similar content being viewed by others
References
Xueqing, Z., Qin, G., Daxiang, C. (2009). Recent advances in nanotechnology applied to biosensors. Sensors, 9, 1033–1053. doi:10.3390/s90201033.
Sherry, L. J., Jin, R., Mirkin, C. A., Schatz, G. C., Van Duyne, R. P. (2006). Localized surface plasmon resonance spectroscopy of single silver triangular nanoprisms. Nano Letters, 6, 2060–2065. doi:10.1021/nl061286u.
Campbell, R. E. (2009). Fluorescent-protein-based biosensors: modulation of energy transfer as a design principle. Analytical Chemistry, 81, 5972–5979. doi:10.1021/ac802613w.
Sahoo, H. (2011). Förster resonance energy transfer—A spectroscopic nanoruler: Principle and applications. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 12, 20–30. doi:10.1016/j.jphotochemrev.2011.05.001.
Sekatskii, S. K. (2004). Fluorescence resonance energy transfer scanning near-field optical microscopy. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 362, 901–919. doi:10.1098/rsta.2003.1354.
Stepensky, D. (2007). FRETcalc plugin for calculation of FRET in non-continuous intracellular compartments. Biochemical and Biophysical Research Communications, 359, 752–758. doi:10.1016/j.bbrc.2007.05.180.
Marques, B. F., & Schneider, J. M. (2006). Effect of electrostatic interactions on binding and retention of DNA oligomers to PNA liposomes assessed by FRET measurements. Colloids and Surfaces. B, Biointerfaces, 53, 1–8. doi:10.1016/j.colsurfb.2006.07.007.
Corry, B., Jayatilaka, D., Rigby, P. (2005). A flexible approach to the calculation of resonance energy transfer efficiency between multiple donors and acceptors in complex geometries. Biophysical Journal, 89, 3822–3836. doi:10.1529/biophysj.105.069351.
Kaláb, P., & Soderholm, J. (2010). The design of Förster (fluorescence) resonance energy transfer (FRET)-based molecular sensors for Ran GTPase. Methods, 51, 220–232. doi:10.1016/j.ymeth.2010.01.022.
Ge, S., Lu, J., Yan, M., Yu, F., Yu, J., Sun, X. (2011). Fluorescence resonance energy transfer sensor between quantum dot donors and neutral red acceptors and its detection of BSA in micelles. Dyes and Pigments, 91, 304–308. doi:10.1016/j.dyepig.2011.05.013.
Jung, J. H., Lee, M. H., Kim, H. J., Jung, H. S., Lee, S. Y., Shin, N. R., No, K., Kim, J. S. (2009). Metal ion induced FRET on–off in naphthyl-pyrenyl pendent tetrahomodioxacalix[4]arene. Tetrahedron Letters, 50, 2013–2016. doi:10.1016/j.tetlet.2009.02.083.
Chen, J., Zheng, A., Chen, A., Gao, Y., He, C., Kai, X., Wu, G., Chen, Y. (2007). A functionalized gold nanoparticles and Rhodamine 6 G based fluorescent sensor for high sensitive and selective detection of mercury (II) in environmental water sample. Analytica Chimica Acta, 599, 134–142. doi:10.1016/j.aca.2007.07.074.
Ziegler, N., Bätz, J., Zabel, U., Lohse, M. J., Hoffmann, C. (2011). FRET-based sensors for the human M1-, M3-, and M5-acetylcholine receptors. Bioorganic & Medicinal Chemistry, 19, 1048–1054. doi:10.1016/j.bmc.2010.07.060.
Giannetti, A., Citti, L., Domenici, C., Tedeschi, L., Baldini, F., Wabuyele, M. B., Vo-Dinh, T. (2006). FRET-based protein–DNA binding assay for detection of active NF-kB. Sensors and Actuators B, 113(2006), 649–654. doi:10.1016/j.snb.2005.07.014.
Cody Stringer, R., Schommer, S., Hoehn, D., Grant, S. A. (2008). Development of an optical biosensor using gold nanoparticles and quantum dots for the detection of porcine reproductive and respiratory syndrome virus. Sensors and Actuators B, 134, 427–431. doi:10.1016/j.snb.2008.05.018
Zhu, J., Yuan, H., Chan, W., Lee, A. W. M. (2010). A FRET fluorescent chemosensor SPAQ for Zn2+ based on a dyad bearing spiropyran and 8-aminoquinoline unit. Tetrahedron Letters, 51, 3550–3554. doi:10.1016/j.tetlet.2010.04.127.
He, G., Zhang, X., He, C., Zhao, X., Duan, C. (2010). Ratiometric fluorescence chemosensors for copper(II) and mercury(II) based on FRET systems. Tetrahedron, 66, 9762–9768. doi:10.1016/j.tet.2010.09.043.
Liu, J., & Lu, Y. (2006). Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nature Protocols, 1, 246–252. doi:10.1038/nprot.2006.38.
Zuo, X., Wu, H., Toh, J., Li, S. F. Y. (2010). Mechanism of mercury detection based on interaction of single-strand DNA and hybridized DNA with gold nanoparticles. Talanta, 82, 1642–1646. doi:10.1016/j.talanta.2010.07.031.
Ou, L., Jin, P. Y., Chu, X., Jiang, J. H., Yu, R. Q. (2010). Sensitive and visual detection of sequence-specific DNA-binding protein via a gold nanoparticle-based colorimetric biosensor. Analytical Chemistry, 82, 6015–6024. doi:10.1038/nprot.2006.38.
Sandstrom, P., Boncheva, M., Åkerman, B. (2003). Nonspecific and thiol-specific binding of DNA to gold nanoparticles. Langmuir, 19, 7537–7543. doi:10.1021/la034348u.
Sandstrom, P., & Akerman, B. (2004). Electrophoretic properties of DNA-modified colloidal gold nanoparticles. Langmuir, 20(2004), 4182–4186. doi:10.1021/la036263z.
Wu, Z. S., Jiang, J. H., Fu, L., Shen, G. L., Yu, R. Q. (2006). Optical detection of DNA hybridization based on fluorescence quenching of tagged oligonucleotide probes by gold nanoparticles. Analytical Biochemistry, 353, 22–29. doi:10.1016/j.ab.2006.01.018.
Fritzsche, W. (2001). DNA-gold conjugates for the detection of specific molecular interactions. Reviews in Molecular Biotechnology, 82, 37–46. doi:10.1016/S1389-0352(01)00028-9.
Zhang, H., Wang, L., Jiang, W. (2011). Label free DNA detection based on gold nanoparticles quenching fluorescence of Rhodamine B. Talanta, 85, 725–729. doi:10.1016/j.talanta.2011.04.057.
Hea, Y., Zhang, X., Zeng, K., Zhang, S., Baloda, M., Gurung, A. S., Liu, G. (2011). Visual detection of Hg2+ in aqueous solution using gold nanoparticles and thymine-rich hairpin DNA probes. Biosensors and Bioelectronics, 26, 4464–4470. doi:10.1016/j.bios.2011.05.003.
Chávez, J. L., Lyon, W., Loughnane, N. K., Stone, M. O. (2010). Theophylline detection using an aptamer and DNA–gold nanoparticle conjugates. Biosensors and Bioelectronics, 26, 23–28. doi:10.1016/j.bios.2010.04.049.
Seidel, C. A. M., Schulz, A., Sauer, M. H. M. (1996). Nucleobase-specific quenching of fluorescent dyes. 1. Nucleobase one-electron redox potentials and their correlation with static and dynamic quenching efficiencies. Journal of Physical Chemistry, 100, 5541–5553. doi:10.1021/jp951507c
Hongslo, J. K., Smith, C. V., Brunborg, G., Søderlund, E. J., Holme, A. J. (1994). Genotoxicity of paracetamol in mice and rats. Mutagenesis, 9, 93–100. doi:10.1093/mutage/9.2.93.
Dybing, E., Holme, J. A., Gordon, W. P., Soderlund, E. J., Dahlin, D. C., Nelson, S. D. (1984). Genotoxicity studies with paracetamol. Mutation Research/Genetic Toxicology, 138, 21–36. doi:10.1016/0165-1218(84)90081-8.
Acknowledgments
The authors would like to acknowledge Cipla Ltd (Mumbai, India) for providing mitoxantrone for monitoring toxicological studies by Nanodevice. We are highly indebted to Bjorn Akerman, Department of Chemistry and Bioscience, Chalmers University of Technology, Kemivagen, Goteborg, Sweden for providing necessary information on DNA–AuNP assembly.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lad, A., Agrawal, Y.K. DNA-Labeled Gold-Based Optical Nanobiosensor Monitoring DNA–Mitoxantrone Interaction. BioNanoSci. 2, 9–15 (2012). https://doi.org/10.1007/s12668-011-0030-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-011-0030-5