Abstract
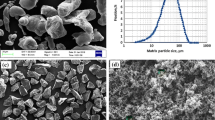
In this research, ball milling of magnesium fillings with NaCl, KCl and urea was investigated as a simple way to produce Mg nanoparticles. Effects of feed geometry and milling time on crush mechanism and product size were determined. Optical, scanning and transmission electron microscopes were used to study the morphology of the products. Spherical charge particles were milled under argon atmosphere with 10 wt% cuboidal NaCl at 250 rpm for 50 h. With ball to powder ratio of 10:1, the average diameter of the product was 17 nm. Addition of NaCl changed the breakage mechanism from ductile–ductile to ductile–brittle, reduced particle size, stopped agglomeration and prevented the undesirable adhesion of particles to walls and balls of the milling machine. Using more than 70 wt% NaCl resulted in conversion of the mechanism from ductile–brittle to brittle–brittle. This resulted in fracture of the particles, but reduced the yield of the system.














Similar content being viewed by others
References
Feldheim D L, and Foss C A, Metal Nanoparticles, Marcel Dekker Inc, Basel (2002).
Kainer K U, Magnesium—Alloys and Technology, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim (2003).
Denisa A, Sellier E, Aymonier C, and Bobet J L, J Alloys Compd 476 (2009) 152.
Song M R, Chen M, and Zhang Z J, Mater Charact 59 (2008) 514.
Bhakta G, Mitra S, and Maitra A, Biomaterials 26 (2005) 2163.
Li C, Cheng F, Ji W, Tao Z, and Chen J, Nano Res 2 (2009) 713.
Samoshina M, Aksenov A, and Kaevitser E, Rev Adv Mater Sci 18 (2008) 305.
Shao H, Wang Y, Xu H, and Li X, Mater Sci Eng B 110 (2004) 221.
Phuoc T X, Howard B H, Martello D V, Soong Y, and Chyu M K, Opt Lasers Eng 46 (2008) 829.
Haas I, and Gedanken A, J R Soc Chem 15 (2008) 1795.
Kooi B J, Palasantzas G, and Hosson J T M D, Appl Phys Lett 89 (2006) 161914.
Rosenkranz S, Breitung-Faes S, and Kwade A, Powder Technol 212 (2011) 224.
Benjamin J S, Met Powder Rep 45 (1990) 122.
Lee P Y, Yang J L, and Lin H M, J Mater Sci 33 (1998) 235.
El-Eskandarany M S, Mechanical Alloying for Fabrication of Advanced Engineering Materials, Noyes, New York (2001).
Suryanarayana C, Prog Mater Sci 46 (2001) 1.
Zhang Y F, Lu L, and Yap S M, J Mater Process Technol 89–90 (1999) 260.
Bodaghi M, Zolfonoon H, Tahriri M, and Karimi M, Solid State Sci 11 (2009) 496.
Alinejad B, and Mahmoodi K, Int J Hydrog Energy 34 (2009) 7934.
Fahimpour V, and Sadrnezhaad S K, Mater Lett 85 (2012) 128.
Zhang Y M, and Kavetsky A, Int J Miner Process 39 (1993) 41.
Fuerstenau D W, Abouzeid A Z M, and Phatak P B, Int J Miner Process 97 (2010) 52.
Ozcan O, and Benzer H, Miner Eng 49 (2013) 103.
Rajamani R K, and Guo D, Int J Miner Process 34 (1992) 103.
Hogg R, Powder Technol 105 (1999) 135.
Acknowledgments
The authors appreciate vice-president of research and technology of Sharif University of Technology for his persuasion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fahimpour, V., Sadrnezhaad, S.K. Breakage Mechanism of Mg During Ball Milling with NaCl, KCl and Urea for Nanopowder Production. Trans Indian Inst Met 70, 1783–1793 (2017). https://doi.org/10.1007/s12666-016-0979-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-016-0979-4