Abstract
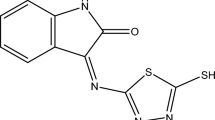
Two synthesized Schiff base derivatives were analyzed as corrosion inhibitors for mild steel in 1 N HCl solution by a series of known techniques such as electrochemical impedance spectroscopy, weight loss, potentiodynamic polarization, linear polarization and scanning electron microscopic at different inhibitor concentrations. The effects demonstrate that two Schiff base derivatives are excellent inhibitors, and inhibition efficiency accepts the order: SB1 > SB2. The adsorption of each inhibitor on metal surface obeys Langmuir adsorption isotherm. Two Schiff base derivatives acted as mixed-type inhibitors. The surface of the mild steel was examined by scanning electron microscope in the absence and presence of Schiff base derivatives.









Similar content being viewed by others
References
Obot I B, and Gasem Z M, Corros Sci 83 (2014) 359.
Sastry V S, Corrosion Inhibitors. Principles and Applications. John Wiley & Sons, New York (1998).
Laila A, Rachid S, Abdelkader Z, Hassan Z, El Houcine B, Belkheir H, and Mohamed Z, Trans Indian Inst Met 66(1) (2013) 43.
Schmitt G, Br Corros J 19 (1984) 165.
Yadav M, Debasis B, Sinha, R R, and Yadav P N, Acta Metall Sinica (English Letters) 27(1) (2014) 37.
Moallem Z, Danaee I, and Eskandari H, Trans Indian Inst Met 67(6) (2014) 817.
Zadeh Hosein A R, Danaee I, and Maddahy M H, J Mater Sci Technol 29(9) (2013) 884.
Ishwara Bhat J, and Vijaya D P A, Trans Indian Inst Met 64(4–5) (2011) 377.
Mistry B M, and Jauhari S, Chem Eng Comm 201 (2014) 961.
Anejjar A, Salghi R., Zarrouk A, Benali O, Zarrok H, Hammouti B, and Ebenso E E, J Assoc Arab Univ Basic Appl Sci 15 (2014) 21.
Trabanelli G, Chemical industries: corrosion mechanism, F. Mansfeld, Marcel Dekker (Ed.), New York (1987), p 120.
Patel N S, Jauhari S, Mehta G N, Hammouti B, Al-Deyab S S, and Bouachrine M, J. Iran. Chem. Soc. 9 (2012) 635.
Karthikaiselvi R, and Subhashini S, J Assoc Arab Univ Basic Appl Sci, (2013) DOI 10.1016/j.jaubas.2013.06.002.
SrinivasaRao S, Appa Rao B V, Roopas Kiran S, and Sreedhar B, J Mater Sci Technol 30(1) (2014) 77.
Afia L, Salghi R, Benali O, Jodeh S, Selim Al-Deyab S, and Hammouti B, Trans Indian Inst Met DOI 10.1007/s12666-014-0479-3.
Mistry B M, and Jauhari S, Res Chem Intermed DOI 10.1007/s11164-014-1740-4.
Lashgaria M, Arshadib M, and Miandaria S, Electrochim Acta 55 (2010) 6058.
Jafari H, Danaee I, and Eskandari H, Trans Indian Inst Met (2015) DOI 10.1007/s12666-014-0506-4.
Yurt A, Bereket G, Kivrak A, Balaban A, and Erk B, J Appl Electrochem 35 (2005) 1025.
Mistry B M, Sahoo S K, Kim D H, and Jauhari S, Surf Interface Anal 47 (2015) 706.
Mohan J P, and Paruthimal Kalaignan G, J Mater Sci Technol 29(11) (2013) 1096.
Mistry B M, and Jauhari S, Med Chem Res 22 (2013) 647.
Barsoukov E, and Macdonald JR, Impedance spectroscopy; theory, experiment, and applications, Second ed., Wiley Interscience Publications, NJ (2005).
Bard A J, and Faulkner L R, Electrochemical methods; fundamentals and applications, Wiley Interscience Publications, NY (2000).
Scully J R, and Silverman D C, Kendig, M.W., (Ed) Electrochemical impedance: analysis and interpretation, ASTM, West Conshohocken, PA (1993).
Mansfeld F, Electrochim. Acta 35 (1990)1533.
Fletcher S, J Electroche Soc 141 (1994) 1823.
Lashgaria M, Arshadi M R, and Biglar M, J Iran Chem Soc 7(2) (2010) 478.
Jafari H, Danaee I, Eskandari H, and RashvandAvei M, J Mater Sci Technol 30(3) (2014) 239.
McCafferty E, and Hackerman N, J Electrochem Soc 119 (1972) 146.
Mistry B M, Sahoo S K, and Jauhari S, J Electroanal Chem 704 (2013) 118.
Li W H, He Q, Zhang S T, Pei C L, and Hou B R, J Appl Electrochem 38 (2008) 289.
Thiraviyam P, and Kannan K, J Iran Chem Soc 9 (2012) 911.
Lebrini M, Lagrenee M, Vezin H, Traisnel M, and Bentiss F, Corros Sci 49 (2007) 2254.
Herrag L, Bouklah M, Patel N S, Mistry B M, Hammouti B, Elkadiri S, and Bouachrine M, Res Chem Intermed 38 (2012) 1669.
Yurt A, Balaban A, Ustun Kandemir S, Bereket G, and Erk B, Mater Chem Phys 85 (2004) 420.
Macdonald J R, and Johnson W B, Thoery in Impedance Spectroscopy, J.R. Macdonald (Eds.), John Wiley & Sons, New York (1987).
Riggs Jr O L, Corrosion inhibitors, C. C. Nathan (Eds.) NACE, USA (1973).
Fu J, Li S, Wang Y, Cao L, and Lu L, J Mater Sci 45 (2010) 6255.
Mohajernia S, Hejazi S, Moayed M H, Rahimizadeh M, Eslami A, Momeni M, and Shiri A, J Iran Chem Soc 10 (2013) 831.
Szauer T, and Brandt A, Electrochim. Acta 26 (1981) 1253.
Aljourani J, Raeissi K, Golozar M A, Corros Sci 51 (2009) 1836.
Donahue F M, and Nobe K, J Electrochem Soc 112 (1965) 886.
Kamis E, Bellucci F, Latanision R M, and El-Ashry E S H, Corrosion 47 (1991) 677.
Mistry B M, Patel N S, Patel M J, Jauhari S, Res Chem Intermed 37 (2011)659.
Mallaiyaa K, Subramaniama R, Srikandana S S, Gowria S, Rajasekaran N, and Selvaraj A, Electrochim Acta 56 (2011) 3857.
Li X, Deng S, Fu H, and Li T, Electrochim Acta 54 (2009) 4089.
Zhao T P, and Mu G N, Corros Sci 41 (1999) 1937.
Bentiss F, Lebrini M, and Lagrenee M, Corros Sci 47(2005) 2915.
Noor E A, and Al-Moubaraki A H, Mater Chem Phys 110 (2008) 145.
Solmaz R, Kardas G, Culha M, Yazici B, and Erbil M, Electrochim Acta 53 (2008) 5941.
Li X, Deng S, Fu H, and Mu G, Corros Sci 51 (2009) 620.
Xu F, Duan J, Zhang S, and Hou B, Mater Lett 62 (2008) 4072.
Mistry B M, Patel N S, Sahoo S, and Jauhari S, Bull Mater Sci 35(3) (2012) 459.
Abd El-Makoud S A, Appl Surf Sci 206 (2003) 129.
Solmaz R, Kardas G, Yazici B, and Erbi, M, Colloid Surf A 312 (2008) 7.
Keles H, Keles M, Dehri I, and Serindag O, Mater Chem Phys 112 (2008) 173.
Acknowledgments
This article was supported by the KU Research Professor Program of Konkuk University, Seoul, South Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mistry, B.M., Kim, D.H. & Jauhari, S. Analysis of Adsorption Properties and Corrosion Inhibition of Mild Steel in Hydrochloric Acid Solution by Synthesized Quinoline Schiff Base Derivatives. Trans Indian Inst Met 69, 1297–1309 (2016). https://doi.org/10.1007/s12666-015-0690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-015-0690-x