Abstract
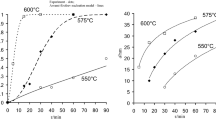
Nanocrystalline ceria powders were prepared by using citrate gel combustion. The influence of the composition of the combustion mixture on the characteristics of the final product was investigated. Ceria powders obtained by calcining the combustion residue in air at 1073 K were characterized for their specific surface area (SSA), X-ray crystallite size (XCS), bulk density (BD), particle size distribution (PSD) and residual carbon. The dependence of these properties on the fuel to oxidant ratio (R) of the initial mixture was investigated. The microstructure of the calcined ceria powders prepared from a mixture with R = 0.25 was investigated by using high resolution transmission electron microscopy. All the calcined powders were pelletised and sintered at 1473, 1673 and 1873 K, and their sinterability was compared by measuring the density of the sintered pellets. A maximum sintered density of 98 % theoretical density could be achieved at a temperature as low as 1473 K for the first time for the powder prepared from a mixture with R = 0.75. The systematic dependence of the properties of these powders on the composition of the initial mixture is being reported for the first time. Powders obtained from a mixture with an R value 0.25 showed a linear increase in sintered densities with the sintering temperature. Other powders exhibited anomalous decrease in the sintered density at high temperature, probably due to irregular grain growth coarsening.








Similar content being viewed by others
References
Patil K C, Hegde M S, Rattan T and Aruna S T, Chemistry of Nanocrystalline Oxide Materials: Combustion Synthesis, Properties and Applications, World Scientific, Singapore (2008).
Tsipis E V and Kharton V V, J Solid State Electrochem 12 (2008) 1039.
Fu Q X, Zha S W, Zhang W, Peng D K, Meng G Y and Zhu B, J Power Sources 104 (2002) 73.
Zhu W Z, and Deevi S C, Mater Sci & Eng A 362 (2003) 228.
Kiork R E and Othmer D F, Encylopedia of Chemistry and Technology, 3rd ed., Wiley, New York, (1979).
Dorr W, Hellmann S, and Mages G, J Nucl Mater 140 (1986) 7.
Jain A, Ananthasivan K, Anthonysamy S, and Rao PRV, J Nucl Mater 345 (2005) 245.
Alifanti M, Baps B, and Blangenois N, Chem Mater 15 (2003) 395.
Zhou Y C, and Rahman M N, J Mater Res 8 (1993) 1680.
Li Y X, Chen W F, and Zhou X Z, Mater Lett 59 (2005) 48.
Yin L X, Wang Y Q, and Pang G S, J Colloid Interface Sci 246 (2002) 78.
Djuricic B, and Pickering S, J Eur Ceram Soc 19 (1999) 1925.
Xu H, Yan H, and Chen Z, J Power Sources 163 (2006) 409.
Palneedi H, Mangam V, Das S, and Das K, J Alloys Compd 509 (2011) 9912.
Hwang C-C, Huang T-H, Tsai J-S, Lin C-S, and Peng C-H, Mater Sci Eng B 132 (2006) 229.
Purohit R D, Sharma B P, Pillai K T, and Tyagi A K, Mater Res Bull 36 (2001) 2711.
Chen W, Li F, Yu J, and Li Y, J Rare Earths 24 (2006) 434.
Gu L, and Meng G, Mater Res Bull 42 (2007) 1323.
Chandramouli V, Anthonysamy S, and Rao PRV, J Nucl Mater 265 (1999) 255.
Anthonysamy S, Ananthasivan K, Chandramouli V, Kaliappan I, and Rao P R V, J Nucl Mater 278 (2000) 346.
Ananthasivan K, Anthonysamy S, Sudha C, Terrance A L E, and Rao P R V, J Nucl Mater 300 (2002) 217.
Biswas M, Prabhakaran K, Gokhale N M, and Sharma S C, Mater Res Bull 42 (2007).
Fu Y-P, and Lin C-H, J Alloys Compd 389 (2005) 165.
Xu H M, Yan H G, and Chen Z H, Mater Character 59 (2008) 301.
Banerjee S, and Devi P S, J Nanopart Res 9 (2007) 1097.
Jain S R, Adiga K C, and Pai Vernekar V R, Combust Flame 40 (1981) 71.
Yuan Q, Duan H-H, Li L-L, Sun L-D, Zhang Y-W, and Yan C-H, Mater Character 335 (2009) 151.
Biswas M, and Bandyopadhyay S, Mater Res Bull 47 (2012) 544.
Biswas M, and Bandyopadhyay S, Adv Powder Technol 25 (2014) 536.
Biswas M, and Bandyopadhyay S, Ceram Int 39 (2013) 9699.
Purohit R D, Saha S, and Tyagi A K, Ceram Int 32 (2006) 143.
Chinarro E, Juradon J R, and Colomer M T, J Eur Ceram Soc 27 (2007) 3619.
Wang X-H, Chen P-L, and Chen I-W, J Am Ceram Soc 89 (2006) 431.
Deng X-Y, Bai H-L, Zhou H, and Chen I-W, J Am Ceram Soc 89 (2006) 438.
Li J-G, Ikegami T, Wang Y, and Mori T, J Am Ceram Soc 85 (2002) 2376.
Dragoo AL, and Domingues L P, J Am Ceram Soc 65 (1982) 253.
Overs A, and Riess I, J Am Ceram Soc 65 (1982) 606.
Higashi K, Sonoda K, Ono H, Sameshima S, and Hirata Y, J Mater Res 14 (1999) 957.
Acknowledgments
The authors would like to acknowledge Dr. G. Panneerselvam for helping to record the X-ray diffraction pattern. The authors would also like to acknowledge Dr. Amirthapandian of Materials Science Group, IGCAR for recording TEM images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balakrishnan, S., Sanjay Kumar, D. & Ananthasivan, K. Gel-Combustion Synthesis of Nanocrystalline Cerium Oxide and Its Powder Characteristics. Trans Indian Inst Met 68 (Suppl 2), 243–252 (2015). https://doi.org/10.1007/s12666-015-0578-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-015-0578-9