Abstract
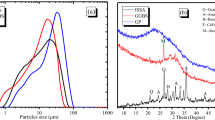
Gaomiaozi (GMZ) bentonite is the most extensively used buffer/backfill material in deep geological repositories used for the disposal of high-level nuclear waste in China. Highly alkaline ground water solutions derived from the cement dissolution may cause changes in the mineralogical composition and in the adsorption capacity of bentonite, and may affect the long-term performance of the engineering barrier system. In the present study, the alteration of montmorillonite and its influence on the adsorptive properties of GMZ bentonite were investigated. The X-ray diffraction patterns of the mineral composition showed that the main compound of the GMZ bentonite is montmorillonite. By contrast, its hydration with a solution of KOH at 80 °C produced cementitious materials, and transformation could likely lead to kaolinisation. Thermodynamic results show that the adsorption of Sr (II) on GMZ bentonite is exothermic and follows quasi-secondary adsorption kinetics. For an initial Sr (II) concentration C0 = 100 mg/L, the maximum amount of Sr (II) adsorbed (qe) on GMZ bentonite was 9.72 mg/g. The adsorption capacity of GMZ bentonite on Sr (II) decreased gradually and as a function of the mineral transformation time in a mixture of GMZ bentonite and KOH. The Freundlich isotherm adsorption model can describe in a satisfactory manner the adsorption of Sr (II) on GMZ bentonite or the transformation of bentonite at different degrees of mineral transformations.














Similar content being viewed by others
Abbreviations
- DGR:
-
Deep geological repository
- GMZ:
-
Gaomiaozi
- HDPE:
-
High-density polyethylene
- HLW:
-
High-level nuclear waste
- ICP–OES:
-
Inductively coupled plasma–optical emission spectrometry
- RPM:
-
Revolutions per minute
- tHM:
-
Tons of heavy metals
- XRD:
-
X-ray diffraction
References
Abdel Raouf MW, El-Kamash AM (2006) Kinetics and thermodynamics of the sorption of uranium and thorium ions from nitric acid solutions onto a TBP impregnated sorbent. J Radioanal Nucl Chem 267:389–395
Ahmadpour A, Zabihi M, Tahmasbi M, Rohani Bastami T (2010) Effect of adsorbents and chemical treatments on the removal of strontium from aqueous solutions. J Hazard Mater 182:552–556
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and smectite: a review. Adv Colloid Interface 140:114–131
Boddu VM, Abburi K, Talbott JL, Smith ED (2003) Removal of hexavalent chromium from wastewater using a new composite chitosan bio-sorbent. Environ Sci Technol 37:4449–4456
Bradbury MH, Baeyens B (2003) Porewater chemistry in compacted re-saturated MX-80 bentonite. J Contam Hydrol 61(1–4):329–338
Chegrouche S, Mellah A, Barkat M (2009) Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination 235:306–318
Chen YG, He Y, Ye WM, Sui WH, Xiao MM (2013) Effect of shaking time, ionic strength, temperature and pH value on desorption of Cr (III) adsorbed onto GMZ bentonite. T Nonferr Metal Soc 23(11):3482–3489
Chen YG, He Y, Ye WM, Jia LY (2015) Competitive adsorption characteristics of Na(I)/Cr(III) and Cu(II)/Cr(III) on GMZ bentonite in their binary solution. J Ind Eng Chem 26:335–339
Chen YG, Sun Z, Cui YJ, Ye WM, Liu QH (2019) Effect of cement solutions on the swelling pressure of compacted GMZ bentonite at different temperatures. Constr Build Mater 229:116872
ENRESA (2000) Full-scale engineered barriers experiment for deep geological repository for high-level radioactive waste in crystalline rock (FEBEX project). EUR 19147 Nucl Sci Technol Series. Luxembourg
Fernández R, Ruiz AI, Cuevas J (2014) The role of smectite composition on the hyperalkaline alteration of bentonite. Appl Clay Sci 95:83–94
Gates WP, Bouazza A (2010) Bentonite transformations in strongly alkaline solutions. Geotext Geomembr 28:219–225
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) Characterizations of strontium (II) and barium (II) adsorption from aqueous solutions using dolomite powder. J Hazard Mater 190:916–921
Guo ZJ, Xu J, Shi KL (2009) Eu (III) adsorption/desorption on Na-bentonite: experimental and modeling studies. Colloids Surf A Physicochem Eng Asp 339:126–133
He Y, Ye WM, Chen YG, Chen B, Ye B, Cui YJ (2016a) Influence of pore fluid concentration on water retention properties of compacted GMZ01 bentonite. Appl Clay Sci 129:131–141
He Y, Chen YG, Ye WM (2016b) Equilibrium, kinetic and thermodynamic studies of adsorption of Sr (II) from aqueous solution onto GMZ bentonite. Environ Earth Sci 75(9):807
He Y, Cui YJ, Ye WM, Conil N (2017) Effects of wetting-drying cycles on the air permeability of compacted Téguline clay. Eng Geol 228:173–179
He Y, Ye WM, Chen YG, Cui YJ (2019a) Effects of K+ solutions on swelling behavior of compacted GMZ bentonite. Eng Geol 249:241–248
He Y, Li BB, Zhang KN, Li Z, Chen YG, Ye WM (2019b) Experimental and numerical study on heavy metal contaminant migration and retention behavior of engineered barrier in tailings pond. Environ Pollut 252:1010–1018
He Y, Ye WM, Chen YG, Zhang KN, Wu DY (2019c) Effects of NaCl solutions on the swelling and shrinkage behavior of compacted bentonite under one-dimensional conditions. Bull Eng Geol Environ. https://doi.org/10.1007/s10064-019-01568-1
He Y, Chen YG, Zhang KN, Ye WM, Wu DY (2019d) Removal of chromium and strontium from aqueous solutions by adsorption on laterite. Arch Environ Prot 45(3):11–20
He Y, Chen YG, Ye WM, Zhang XX (2020) Effects of contact time, pH, and temperature on Eu(III) sorption onto MX-80 bentonite. Chem Phys 534:110742
Herbert HJ, Kasbohm J, Sprenger H, Fernández AM, Reichelt C (2008) Swelling pressures of MX-80 bentonite in solutions of different ionic strength. Phys Chem Earth 33:S327–S342
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ Prot 76:183–191
Hurel C, Marmier N (2010) Sorption of europium on a MX-80 bentonite sample: experimental and modelling results. J Radioanal Nucl Chem 284:225–230
Japan Nuclear Cycle Development Institute (JNC) (2000) Japan Nuclear Cycle Development Institute (JNC), H12: Project to establish the technical basis for HLW disposal in Japan. Supporting Report 3, Safety Assessment of the Geological Disposal System, JNC Tech. Rep., JNC TN1401 2000–2004
Karnland O, Birgersson M (2006) Montmorillonite stability with special respect to KBS-3 conditions, SKB Technical Report TR-06–11, Swedish Nuclear Fuel and Waste Management Company, Stockholm, Sweden.
Kurosawa S, Ueta S (2001) Effect of colloids on radionuclide migration for performance assessment of HLW disposal in Japan. Pure Appl Chem 73(12):2027–2037
Lehikoinen J (2009) Bentonite-cement interaction. Preliminary results from model calculations, Posiva Working Rep. 2009–37. Posiva Oy, Eurajoki, Helsinki, Finland.
Liu L, Luo X, Ding L, Luo S (2019) Application of nanotechnology in the removal of heavy metal from water, micro and nano technologies. Elsevier, Amsterdam, pp 83–147
Lu C, Samper J, Cormenzana JL, Ma HY, Montenegro L, Cunñado MÁ (2012) Reactive transport model and apparent Kd of Ni in the near field of a HLW repository in granite. Comput Geosci-UK 49:256–266
Marcial D, Delage P, Cui YJ (2002) On the high stress compression of bentonites. Can Geotech J 39:812–820
Martín PL, Barcala JM, Huertas F (2006) Large-scale and long-term coupled thermo-hydro-mechanic experiments with bentonite: the FEBEX mock-up test. J Iber Geol 32:259–282
Missana T, García-Gutierrez M (2007) Adsorption of bivalent ions (Ca (II), Sr (II) and Co (II)) onto FEBEX bentonite. Phys Chem Earth 32:559–567
Mohanty JK, Guru SR, Dash P, Pradhan PK (2021) Fly ash management and condition monitoring of ash pond. Earth Syst Environ 5:445–457
Montavon G, Alhajji E, Grambow B (2006) Study of the interaction of Ni2+ and Cs+ on MX-80 bentonite: effect of compaction using the “capillary method.” Environ Sci Technol 40:4672–4679
Nabbou N, Belhachemi M, Boumelik M, Merzougui T, Lahcene D, Harek Y, Zorpas AA, Jeguirim M (2019) Removal of fluoride from groundwater using natural clay (kaolinite): optimization of adsorption conditions. C R Chim 22(2–3):105–112
Nakayama S, Sakamoto Y, Yamaguchi T, Akai M, Tanaka T, Sato T, Iida Y (2004) Dissolution of smectite in compacted bentonite by highly alkaline aqueous solutions and diffusivity of hydroxide ions. Appl Clay Sci 27(1–2):53–65
Ӧzcan AS, Gök Ӧ, Ӧzcan A (2009) Adsorption of lead (II) ions onto 8-hydroxy quinoline-immobilized bentonite. J Hazard Mater 161:499–509
POSIVA (2015) Annual report, 2015. http://www.posiva.fi/files/4276/Posiva-Vuosikertomus2015-EN.pdf
Pusch R, Karnland O, Sandén T (1996) Final report on physical testing programme concerning Spanish clays (saponites and bentonites), Enresa Tech. Rep. 02/96.
Reddad Z, Gerente C, Andres Y, Le Cloirec P (2002) Adsorption of several metal ions onto a low-cost bio-sorbent: kinetic and equilibrium studies. Environ Sci Technol 36:2067–2073
Sato T, Kuroda M, Yokoyama S, Tsutsui M, Pacau C, Ringor C, Fukushi K, Tanaka T, Nakayama S (2005) Dissolution kinetics of smectite under alkaline conditions. In: International meeting: Clays in natural and engineering barriers for nuclear waste confinement, March 14–18, 2005, Tours, France.
Savage D, Benbow S (2007) Low-pH cements. Swedish nuclear power inspectorate. SKI Rep. 2007:32. Swedish Nuclear Power Inspectorate (Stockholm), 2007.
Svensk Kärnbränslehantering AB (SKB) (1999) SR-97. Waste, repository design and sites. SKB Technical Report TR-99-08, Stockholm, Sweden, p 90
Tahir SS, Rauf N (2003) Thermodynamic studies of Ni (II) adsorption onto bentonite from aqueous solution. J Chem Thermodyn 35:2003–2009
Wang SW, Hu J, Li JX, Dong YH (2009) Influence of pH, soil humic/fulvic acid, ionic strength, foreign ions and addition sequences on adsorption of Pb (II) onto GMZ bentonite. J Hazard Mater 167:44–51
Wang J (2013) On area-specific underground research laboratory for geological disposal of high-level radioactive waste in China. In: Third international symposium on unsaturated soil mechanics and deep geological waste disposal, 7–10 July 2013, Shanghai, China.
Wang J (2017) Geological disposal of high-level radioactive waste in China and plan for URL. In: 4th international symposium on unsaturated soil mechanics and waste disposal, 15–16 July 2017 Shanghai, China.
Wen ZJ (2005) Selection and basic properties of China’s buffer materials for high level radioactive waste repository. Acta Petrol Et Mineral 24(6):583–586 ((in Chinese))
Wen ZJ (2008) Selection and basic properties of the buffer material for high-level radioactive waste repository in China. Acta Geol Sin 82:1050–1055
Wood M (1983). Experimental investigation of sodium bentonite stability in Hanford basalt. In: Bookins DC (ed) Material research society symposium, Amsterdam, 15:727–743.
Xu D, Xu XL, Tan XL, Chen CL, Wang XK (2008) Adsorption of Pb (II) from aqueous solution to MX-80 bentonite: Effect of pH, ionic strength, foreign ions and temperature. Appl Clay Sci 41:37–46
Yang ST, Li JX, Lu Y, Chen YX, Wang XK (2009) Sorption of Ni (II) on GMZ bentonite: effects of pH, ionic strength, foreign ions, humic acid and temperature. Appl Radiat Isot 67:1600–1608
Ye WM, Chen YG, Chen B, Wang Q, Wang J (2010) Advances on the knowledge of the buffer/backfill properties of heavily-compacted GMZ bentonite. Eng Geol 116(27):12–20
Ye WM, Wan M, Chen B, Chen YG, Cui YJ, Wang J (2012) Temperature effects on the unsaturated permeability of the densely compacted GMZ01 bentonite under confined conditions. Eng Geol 126:1–7
Ye WM, He Y, Chen YG, Chen B, Cui YJ (2016) Thermochemical effects on the smectite alteration of GMZ bentonite for deep geological repository. Environ Earth Sci 75(10):906
Zhao DL, Feng SJ, Chen CL, Chen SH, Xu D, Wang XK (2008) Adsorption of Thorium (IV) on MX-80 bentonite: effect of pH ionic strength and temperature. Appl Clay Sci 41:17–23
Zhao DL, Chen SH, Yang SB, Yang X, Yang ST (2011) Investigation of the sorption behavior of Cd (II) on GMZ bentonite as affected by solution chemistry. Chem Eng J b166:1010–1016
Acknowledgements
The authors thank the National Natural Science Foundation of China (Projects 42072318 and 41972282) and the Natural Science Foundation of Hunan Province, China (Project 2019JJ50763) for their financial support. The authors also thank the Financial support from National Key Research and Development Program of China (No. 2019YFC1803600).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Yy., He, Y., Zhang, Kn. et al. Montmorillonite alteration and its influence on Sr (II) adsorption on GMZ bentonite. Environ Earth Sci 80, 791 (2021). https://doi.org/10.1007/s12665-021-10093-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-10093-y