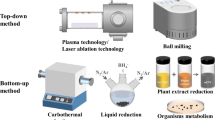
Abstract
Africa has the second largest ultimately recoverable resources (URR) of gigaton (Gt) iron (Fe) ore in the world. South Africa is among the countries in the world with high-quality iron ore deposits. The most significant sources of Fe ore in the African continent are hosted by the banded iron ore formation of Precambrian age with high-quality hematite (Fe2O3) bodies. Synthetic magnetite (Fe3O4) nanoparticles (NPs), in recent years, have emerged as novel magnetic NPs with structurally distinct properties because of intensive research efforts by many researchers. They exhibited almost the same chemical and physical properties with naturally occurring Fe3O4, and they have been successfully adopted in removing trace metals from groundwater. For a better understanding of the development of suitable techniques for their application in contaminated groundwater, this paper emphasizes the synthetic methodology, surface modification, coating with porous materials/inorganic clays, functionalized composites, properties, and its ex situ groundwater remediation application. Magnetic separation is the most suitable and cost-effective method for the groundwater remediation of trace metals for both high and lower concentrations.













Similar content being viewed by others
References
Abdeen M, Sabry S, Ghozlan H, El-Gendy AA, Carpenter EE (2016) Microbial-physical synthesis of Fe and Fe3O4 magnetic nanoparticles using Aspergillus niger YESM1 and supercritical condition of ethanol. J Nanomaterials, 2016
Absar N, Sreenivas B (2015) Petrology and geochemistry of greywackes of the ~ 1.6 Ga middle Aravalli supergroup, Northwest India: evidence for active margin processes. Int Geol Rev 57(2):134–158
Ajaka E (2009) Recovering fine iron minerals from Itakpe iron ore process tailing. ARPN J Eng Appl Sci 4(9):1–28
Akl M, Yousef A, AbdElnasser S (2013) Removal of iron and manganese in water samples using activated carbon derived from local agro-residues. J Chem Eng Process Technol 4:4. https://doi.org/10.4172/2157-7048.1000154
Aliyari E, Alvand M, Shemirani F (2016) Modified surface-active ionic liquid-coated magnetic graphene oxide as a new magnetic solid phase extraction sorbent for preconcentration of trace nickel. RSC Adv 6(69):64193–64202
Al-Jabri MTK, Devi MG, Al Abri M (2018) Synthesis, characterization and application of magnetic nanoparticles in the removal of copper from aqueous solution. Appl Water Sci 8(8):223
Ambashta RD, Sillanpää M (2010) Water purification using magnetic assistance: a review. J Hazard Mater 180(1–3):38–49
Apukhtina OB, Kamenetsky VS, Ehrig K, Kamenetsky MB, McPhie J, Maas R, Cook NJ (2016) Postmagmatic magnetite–apatite assemblage in mafic intrusions: a case study of dolerite at Olympic Dam, South Australia. Contrib Miner Petrol 171(1):2
Arenas-Alatorre J, Lukas O, Rodríguez-Gómez A, Reyes RH, Tapia-del León C (2019) Synthesis and characterization of iron oxide nanoparticles grown via a non-conventional chemical method using an external magnetic field. Mater Lett 242:13–16
Bagheri H, Afkhami A, Saber-Tehrani M, Khoshsafar H (2012) Preparation and characterization of magnetic nanocomposite of Schiff base/silica/magnetite as a preconcentration phase for the trace determination of heavy metal ions in water, food and biological samples using atomic absorption spectrometry. Talanta 97:87–95
Bai H, Sheng K, Zhang P, Li C, Shi G (2011) Graphene oxide/conducting polymer composite hydrogels. J Mater Chem 21(46):18653–18658
Bao S, Tang L, Li K, Ning P, Peng J, Guo H, Liu Y (2016) Highly selective removal of Zn (II) ion from hot-dip galvanizing pickling waste with amino-functionalized Fe3O4@ SiO2 magnetic nano-adsorbent. J Colloid Interface Sci 462:235–242
Basson I, Koegelenberg C (2017) Structural controls on Fe mineralization at Thabazimbi mine, South Africa. Ore Geol Rev 80:1056–1071
Beukes N, Gutzmer J, Mukhopadhyay J (2003) The geology and genesis of high-grade hematite iron ore deposits. Appl Earth Sci 112(1):18–25
Bin Jusoh A, Cheng W, Low W, Nora’aini A, Noor MMM (2005) Study on the removal of iron and manganese in groundwater by granular activated carbon. Desalination 182(1–3):347–353
Bortey-Sam N, Nakayama SM, Ikenaka Y, Akoto O, Baidoo E, Mizukawa H, Ishizuka M (2015) Health risk assessment of heavy metals and metalloid in drinking water from communities near gold mines in Tarkwa, Ghana. Environ Monit Assess 187(7):397
Boutroy E, Dare SA, Beaudoin G, Barnes SJ, Lightfoot PC (2014) Magnetite composition in Ni–Cu–PGE deposits worldwide: application to mineral exploration. J Geochem Explor 145:64–81
Cao W, Ma Y, Zhou W, Guo L (2015) One-pot hydrothermal synthesis of rGO-Fe3O4 hybrid nanocomposite for removal of Pb(II) via magnetic separation. Chem Res Chin Univ 31(4):508–513
Carlut J, Isambert A, Bouquerel H, Pecoits E, Philippot P, Vennin E, Baton F (2015) Low temperature magnetic properties of the Late archean boolgeeda iron formation (Hamersley Group, Western Australia): environmental implications. Front Earth Sci 3:18
Chandra V, Park J, Chun Y, Lee JW, Hwang IC, Kim KS (2010) Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 4(7):3979–3986
Chang L, Heslop D, Roberts AP, Rey D, Mohamed KJ (2016) Discrimination of biogenic and detrital magnetite through a double Verwey transition temperature. J Geophys Res 121(1):3–14
Chen H, Wei G, Han X, Li S, Wang P, Chubik M, Han W (2011) Large-scale synthesis of hierarchical alpha-FeOOH flowers by ultrasonic-assisted hydrothermal route. J Mater Sci 22(3):252–259
Chowdhury SR, Yanful EK (2010) Arsenic and chromium removal by mixed magnetite–maghemite nanoparticles and the effect of phosphate on removal. J Environ Manage 91(11):2238–2247
Chowdhury S, Mazumder MJ, Al-Attas O, Husain T (2016) Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ 569:476–488
Çiftçi H, Ersoy B, Evcin A (2017) Synthesis, characterization and Cr(VI) Adsorption properties of modified magnetite nanoparticles. Acta Phys Polonica A 132(3):564–569
Crane R, Scott T (2012) Nanoscale zero-valent iron: prospects for an emerging water treatment technology. J Hazard Mater 211:112–125
Cui L, Wang Y, Gao L, Hu L, Yan L, Wei Q, Du B (2015) EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu (II) in water treatment: adsorption mechanism and separation property. Chem Eng J 281:1–10
Dare SA, Barnes SJ, Beaudoin G (2015) Did the massive magnetite “lava flows” of El Laco (Chile) form by magmatic or hydrothermal processes? New constraints from magnetite composition by LA–ICP–MS. Miner Deposita 50(5):607–617
Davodi B, Jahangiri M, Ghorbani M (2019) Magnetic Fe3O4@ polydopamine biopolymer: synthesis, characterization and fabrication of promising nanocomposite. J Vinyl Add Tech 25(1):41–47
de Groot LV, Dekkers MJ, Visscher M, ter Maat GW (2014) Magnetic properties and paleointensities as function of depth in a Hawaiian lava flow. Geochem Geophys Geosyst 15(4):1096–1112
de Mendonça ESDT, de Faria ACB, Dias SCL, Aragón FF, Mantilla JC, Coaquira JA, Dias JA (2019) Effects of silica coating on the magnetic properties of magnetite nanoparticles. Surf Interfaces 14:34–43
de Sousa DV, Ker JC, Schaefer CER, Rodet MJ, Guimarães LM, Felix JF (2018) Magnetite originating from bonfires in a Brazilian prehistoric Anthrosol: a micro-Raman approach. CATENA 171:552–564
Deng H, Li X, Peng Q, Wang X, Chen J, Li Y (2005) Monodisperse magnetic single-crystal ferrite microspheres. Angew Chem 117(18):2842–2845
Dill H (2007) Grain morphology of heavy minerals from marine and continental placer deposits, with special reference to Fe–Ti oxides. Sed Geol 198(1–2):1–27
Dong YZ, Choi HJ (2018) Synthesis of organic-inorganic poly (diphenylamine)/magnetite composite particles and their magnetorheological response. IEEE Trans Magn 99:1–4
Dong H, Xing C, Wang CY (2013) Textures and mineral compositions of the Xinjie layered intrusion, SW China: implications for the origin of magnetite and fractionation process of Fe–Ti-rich basaltic magmas. Geosci Front 4(5):503–515
Dong Z, Zhang F, Wang D, Liu X, Jin J (2015) Polydopamine-mediated surface-functionalization of graphene oxide for heavy metal ions removal. J Solid State Chem 224:88–93
Ebrahimpour B, Yamini Y, Seidi S, Tajik M (2015) Nano polypyrrole-coated magnetic solid phase extraction followed by dispersive liquid phase microextraction for trace determination of megestrol acetate and levonorgestrel. Anal Chim Acta 885:98–105
Elshater R, Kawamura G, Fakhry F, Meaz T, Amer M, Matsuda A (2019) Structural phase transition of spinel to hematite of as-prepared Fe2+-Cr nanoferrites by sintering temperature. Measurement 132:272–281
Equities O (2011) Differentiating amongst the African juniors and identifying the next serious contenders. Iron ore sector update, 7
Etim R, Eberemu A, Osinubi K (2017) Stabilization of black cotton soil with lime and iron ore tailings admixture. Transportation Geotech 10:85–95
Faaliyan K, Abdoos H, Borhani E, Afghahi SSS (2018) Magnetite-silica nanoparticles with core-shell structure: single-step synthesis, characterization and magnetic behavior. J Sol Gel Sci Technol 88(3):609–617
Fan HL, Zhou SF, Jiao WZ, Qi GS, Liu YZ (2017) Removal of heavy metal ions by magnetic chitosan nanoparticles prepared continuously via high-gravity reactive precipitation method. Carbohyd Polym 174:1192–1200
Faraji M, Yamini Y, Rezaee M (2010) Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. J Iran Chem Soc 7(1):1–37
Farimani MHR, Shahtahmasebi N, Roknabadi MR, Ghows N, Kazemi A (2013) Study of structural and magnetic properties of superparamagnetic Fe3O4/SiO2 core–shell nanocomposites synthesized with hydrophilic citrate-modified Fe3O4 seeds via a sol–gel approach. Phys E 53:207–216
Feng Y, Du Y, Chen Z, Du M, Yang K, Lv X, Li Z (2018) Synthesis of Fe3O4 nanoparticles with tunable sizes for the removal of Cr(VI) from aqueous solution. J Coat Technol Res 15(5):1145–1155
Fu R, Liu Y, Lou Z, Wang Z, Baig SA, Xu X (2016) Adsorptive removal of Pb(II) by magnetic activated carbon incorporated with amino groups from aqueous solutions. J Taiwan Inst Chem Eng 62:247–258
Galindo-Gonzalez C, De Vicente J, Ramos-Tejada M, Lopez-Lopez M, Gonzalez-Caballero F, Duran J (2005) Preparation and sedimentation behavior in magnetic fields of magnetite-covered clay particles. Langmuir 21(10):4410–4419
Gavaskar A, Tatar L, Condit W (2005) Cost and performance report nanoscale zero-valent iron technologies for source remediation (No. CR- 05-007-ENV). In: Naval facilities engineering service center Port Hueneme CA
Ghazanfari MR, Kashefi M, Shams SF, Jaafari MR (2016) Perspective of Fe3O4 nanoparticles role in biomedical applications. Biochem Res Int, 2016
Ghezzi L, D’Orazio M, Doveri M, Lelli M, Petrini R, Giannecchini R (2019) Groundwater and potentially toxic elements in a dismissed mining area: thallium contamination of drinking spring water in the Apuan Alps (Tuscany, Italy). J Geochem Explor 197:84–92
Giraldo L, Erto A, Moreno-Piraján JC (2013) Magnetite nanoparticles for removal of heavy metals from aqueous solutions: synthesis and characterization. Adsorption 19(2–4):465–474
Girginova PI, Daniel-da-Silva AL, Lopes CB, Figueira P, Otero M, Amaral VS, Trindade T (2010a) Silica coated magnetite particles for magnetic removal of Hg2 + from water. J Colloid Interface Sci 345(2):234–240
Girginova PI, Daniel-da-Silva AL, Lopes CB, Figueira P, Otero M, Amaral VS, Trindade T (2010b) Silica coated magnetite particles for magnetic removal of Hg2+ from water. J Colloid Interface Sci 345(2):234–240
Grant F (1985) Aeromagnetics, geology and ore environments, I. Magnetite in igneous, sedimentary and metamorphic rocks: an overview. Geoexploration 23(3):303–333
Grau-Atienza A, Serrano E, Linares N, Svedlindh P, Seisenbaeva G, Garcia-Martinez J (2016) Magnetically separable mesoporous Fe3O4/silica catalysts with very low Fe3O4 content. J Solid State Chem 237:138–143
Hamza MF, Wei Y, Mira H, Adel AH, Guibal E (2019) Synthesis and adsorption characteristics of grafted hydrazinyl amine magnetite-chitosan for Ni (II) and Pb(II) recovery. Chem Eng J 362:310–324
Harmsworth R, Kneeshaw M, Morris R, Robinson C, Shrivastava P (1990) BIF-derived iron ores of the Hamersley Province. Geol Miner Depos Aust P N G 1:617–642
Hashemian S, Saffari H, Ragabion S (2015) Adsorption of cobalt (II) from aqueous solutions by Fe 3 O 4/bentonite nanocomposite. Water Air Soil Pollut 226(1):2212
Hu YL, Gao JQ (2010) Potential neurotoxicity of nanoparticles. Int J Pharm 394(1–2):115–121
Hu J, Lo I, Chen G (2004) Removal of Cr(VI) by magnetite. Water Sci Technol 50(12):139–146
Hu C, Guo J, Zhong H, Chen Z, Wen J (2016) The spherical α-Fe2O3 nanomaterials prepared by hydrolysis route and hydrothermal route for lithium-ion batteries. Mater Res Innov 20(1):76–80
Huang Q, Shi X, Pinto RA, Petersen EJ, Weber WJ Jr (2008) Tunable synthesis and immobilization of zero-valent iron nanoparticles for environmental applications. Environ Sci Technol 42(23):8884–8889
Huang XW, Zhou MF, Beaudoin G, Gao JF, Qi L, Lyu C (2018) Origin of the volcanic-hosted Yamansu Fe deposit, eastern Tianshan, NW China: constraints from pyrite Re-Os isotopes, stable isotopes, and in situ magnetite trace elements. Miner Deposita 53(7):1039–1060
Hunt CP, Moskowitz BM, Banerjee SK (1995) Magnetic properties of rocks and minerals. Rock Phys Phase Relations 3:189–204
Hurst L (2013) West and central African iron ore development and its impact on world prices. Aust J Agric Resource Econ 57(4):521–538
Hurst L (2015) Assessing the competitiveness of the supply side response to China’s iron ore demand shock. Resour Policy 45:247–254
Immink H, Louw R, Brent A (2018) Tracking decarbonisation in the mining sector. J Energy South Afr 29(1):14–23
Jankovský O, Marvan P, Nováček M, Luxa J, Mazanek V, Klímová K, Sofer Z (2016) Synthesis procedure and type of graphite oxide strongly influence resulting graphene properties. Appl Mater Today 4:45–53
Javanbakht V, Ghoreishi SM, Habibi N, Javanbakht M (2016) A novel magnetic chitosan/clinoptilolite/magnetite nanocomposite for highly efficient removal of Pb(II) ions from aqueous solution. Powder Technol 302:372–383
Javanbakht V, Ghoreishi SM, Habibi N, Javanbakht M (2017) Synthesis of zeolite/magnetite nanocomposite and a fast-experimental determination of its specific surface area. Protection Metals Phys Chem Surf 53(4):693–702
Jin X, Wu X, Zhang Z, Huang Z, Liu YG, Fang M, Min X (2018) Preparation of carbon-coated Fe3O4 porous particles and their adsorption properties of iron (III) ion. Water Sci Technol 18(1):306–317
Kakavandi B, Kalantary RR, Jafari AJ, Nasseri S, Ameri A, Esrafili A, Azari A (2015) Pb(II) adsorption onto a magnetic composite of activated carbon and superparamagnetic Fe3O4 nanoparticles: experimental and modeling study. Clean-Soil Air Water 43(8):1157–1166
Kampalanonwat P, Supaphol P (2010) Preparation and adsorption behavior of aminated electrospun polyacrylonitrile nanofiber mats for heavy metal ion removal. ACS Appl Mater Interfaces 2(12):3619–3627
Kang BK, Lim BS, Yoon Y, Kwag SH, Park WK, Song YH, Yoon DH (2017) Efficient removal of arsenic by strategically designed and layer-by-layer assembled PS@ + rGO@ GO@ Fe3O4 composites. J Environ Manage 201:286–293
Kataria N, Garg V (2018) Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: regeneration and mechanism. Chemosphere 208:818–828
King VT (1977) The study of minerals. Rocks Miner 52(6):331–333
Koo KN, Ismail AF, Othman MHD, Bidin N, Rahman MA (2019) Preparation and characterization of superparamagnetic magnetite (Fe3O4) nanoparticles: a short review. Malays J Fundam Appl Sci 15(2019):23–31
Kose T, Gharde B, Gholse S (2012) Studies on Albizia procera legumes for effective removal of Fe(II) and Mn(II) from aqueous solution. J Chem Pharm Res 4(4):2021–2028
Kumar R, Sakthivel R, Behura R, Mishra B, Das D (2015) Synthesis of magnetite nanoparticles from mineral waste. J Alloy Compd 645:398–404
Kumari M, Pittman CU Jr, Mohan D (2015) Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J Colloid Interface Sci 442:120–132
Kuşcu M, Cengiz O, Işık K, Gül EK (2018) The origin and geochemical characteristics of rutile in eluvial and fluvial-alluvial placers and quartz veins of the menderes massif from the neoproterozoic Pan-African Belt, western Turkey. J Afr Earth Sc 143:10–27
Lankford Jr, WT, Samways NL, Craven RF, McGannon HE (1985) The making, shaping, and treating of steel. 10 edn. Association of Iron and Steel Engineering, Pittsburgh, PA
Lasheen M, El-Sherif IY, Sabry DY, El-Wakeel S, El-Shahat M (2016) Adsorption of heavy metals from aqueous solution by magnetite nanoparticles and magnetite-kaolinite nanocomposite: equilibrium, isotherm and kinetic study. Desalin Water Treat 57(37):17421–17429
Lassoued A, Lassoued MS, Dkhil B, Ammar S, Gadri A (2018) Synthesis, photoluminescence and magnetic properties of iron oxide (α-Fe2O3) nanoparticles through precipitation or hydrothermal methods. Phys E 101:212–219
Lattard D, Engelmann R, Kontny A, Sauerzapf U (2006) Curie temperatures of synthetic titanomagnetites in the Fe–Ti–O system: Effects of composition, crystal chemistry, and thermomagnetic methods. J Geophys Res. https://doi.org/10.1029/2006JB004591
Lin HJ, Sung TI, Chen CY, Guo HR (2013) Arsenic levels in drinking water and mortality of liver cancer in Taiwan. J Hazard Mater 262:1132–1138
Linnikov O, Krasil’nikov V, Gyrdasova O, Rodina I, Baklanova I, Tyutyunnik A, Marchenkov V (2018) Precursor synthesis of maghemite and its adsorption properties with respect to bivalent copper ions. Adsorption 24(7):629–636
Liu P, Liu Y, Su Z (2006) Modification of poly (hydroethyl acrylate)-grafted cross-linked poly (vinyl chloride) particles via surface-initiated atom-transfer radical polymerization (SI-ATRP) Competitive adsorption of some heavy metal ions on modified polymers. Ind Eng Chem Res 45(7):2255–2260
Liu X, Hu Q, Fang Z, Zhang X, Zhang B (2008) Magnetic chitosan nanocomposites: a useful recyclable tool for heavy metal ion removal. Langmuir 25(1):3–8
Liu PP, Zhou MF, Chen WT, Gao JF, Huang XW (2015) In-situ LA–ICP–MS trace elemental analyses of magnetite: Fe–Ti–(V) oxide-bearing mafic–ultramafic layered intrusions of the Emeishan Large Igneous Province, SW China. Ore Geol Rev 65:853–871
Liu J, Yang H, Xue X (2018) A new and simple route to prepare γ-Fe2O3 with iron oxide scale. Mater Lett 229:156–159
Lu AH, Salabas EEL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46(8):1222–1244
Lu T, Wang J, Yin J, Wang A, Wang X, Zhang T (2013) Surfactant effects on the microstructures of Fe3O4 nanoparticles synthesized by microemulsion method. Colloids Surf A 436:675–683
Lyons R, Oldfield F, Williams E (2010) Mineral magnetic properties of surface soils and sands across four North African transects and links to climatic gradients. Geochem Geophys Geosystems. https://doi.org/10.1029/2010GC003183
Madadrang CJ, Kim HY, Gao G, Wang N, Zhu J, Feng H, Hou S (2012) Adsorption behavior of EDTA-graphene oxide for Pb(II) removal. ACS Appl Mater Interfaces 4(3):1186–1193
Mahdavi M, Ahmad MB, Haron MJ, Gharayebi Y, Shameli K, Nadi B (2013) Fabrication and characterization of SiO2/(3-aminopropyl) triethoxysilane-coated magnetite nanoparticles for lead (II) removal from aqueous solution. J Inorg Organomet Polym Mater 23(3):599–607
Maiti D, Aravindan V, Madhavi S, Devi PS (2015) Electrochemical performance of hematite nanoparticles derived from spherical maghemite and elongated goethite particles. J Power Sources 276:291–298
Makvandi S, Ghasemzadeh-Barvarz M, Beaudoin G, Grunsky EC, McClenaghan MB, Duchesne C, Boutroy E (2016) Partial least squares-discriminant analysis of trace element compositions of magnetite from various VMS deposit subtypes: application to mineral exploration. Ore Geol Rev 78:388–408
Makvandi S, Beaudoin G, McClenaghan MB, Quirt D (2017) Geochemistry of magnetite and hematite from unmineralized bedrock and local till at the Kiggavik uranium deposit: implications for sediment provenance. J Geochem Explor 183:1–21
Malaidurai M, Thangavel R (2018) Study of structural and magnetic properties of co-precipitated α-Fe2O3 nanocrystals. Superlattices Microstruct 120:553–560
Mao J, Xie G, Duan C, Pirajno F, Ishiyama D, Chen Y (2011) A tectono-genetic model for porphyry–skarn–stratabound Cu–Au–Mo–Fe and magnetite–apatite deposits along the Middle-Lower Yangtze River Valley, Eastern China. Ore Geology Reviews 43(1):294–314
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4(8):4806–4814
Mariño-Fernández RA, Masunaga SH, Fontaíña-Troitiño N, Morales MP, Rivas J, Salgueirino V (2011) Goethite (α-FeOOH) nanorods as suitable antiferromagnetic substrates. J Phys Chem C 115(29):13991–13999
Markets RBC (2011) African iron ore projects: potential for new supply. RBC Prospector, 4
Maxbauer DP, Feinberg JM, Fox DL, Clyde WC (2016) Magnetic minerals as recorders of weathering, diagenesis, and paleoclimate: a core–outcrop comparison of Paleocene-Eocene paleosols in the Bighorn Basin, WY, USA. Earth Planet Sci Lett 452:15–26
Mirabi A, Rad AS, Nourani S (2015) Application of modified magnetic nanoparticles as a sorbent for preconcentration and determination of nickel ions in food and environmental water samples. TrAC Trends Anal Chem 74:146–151
Mittal A, Ahmad R, Hasan I (2016) Poly (methyl methacrylate)-grafted alginate/Fe3O4 nanocomposite: synthesis and its application for the removal of heavy metal ions. Desalin Water Treat 57(42):19820–19833
Mohammed L, Gomaa HG, Ragab D, Zhu J (2017) Magnetic nanoparticles for environmental and biomedical applications: a review. Particuology 30:1–14
Mohr S, Giurco D, Yellishetty M, Ward J, Mudd G (2015) Projection of iron ore production. Nat Resour Res 24(3):317–327
Molaei K, Bagheri H, Asgharinezhad AA, Ebrahimzadeh H, Shamsipur M (2017) SiO2-coated magnetic graphene oxide modified with polypyrrole–polythiophene: a novel and efficient nanocomposite for solid phase extraction of trace amounts of heavy metals. Talanta 167:607–616
Murugadoss S, Lison D, Godderis L, Van Den Brule S, Mast J, Brassinne F, Hoet PH (2017) Toxicology of silica nanoparticles: an update. Arch Toxicol 91(9):2967–3010
Musico YLF, Santos CM, Dalida MLP, Rodrigues DF (2013) Improved removal of lead (II) from water using a polymer-based graphene oxide nanocomposite. J Mater Chem A 1(11):3789–3796
Nadoll P, Angerer T, Mauk JL, French D, Walshe J (2014) The chemistry of hydrothermal magnetite: a review. Ore Geol Rev 61:1–32
Nethaji S, Sivasamy A, Mandal A (2013) Preparation and characterization of corn cob activated carbon coated with nano-sized magnetite particles for the removal of Cr(VI). Biores Technol 134:94–100
Nie L, Zhou T, Fan Y, Zhang L, Cooke D, White N (2017) Geology, geochemistry and genesis of the makou magnetite-apatite deposit in the Luzong volcanic basin, Middle–Lower Yangtze River Valley metallogenic belt, eastern China. Ore Geol Rev 91:264–277
Nodeh HR, Ibrahim WAW, Ali I, Sanagi MM (2016) Development of magnetic graphene oxide adsorbent for the removal and preconcentration of As (III) and As (V) species from environmental water samples. Environ Sci Pollut Res 23(10):9759–9773
Oldfield F, Hao Q, Bloemendal J, GIBBS-EGGAR Z, Patil S, Guo Z (2009) Links between bulk sediment particle size and magnetic grain-size: general observations and implications for Chinese loess studies. Sedimentology 56(7):2091–2106
Pasandideh EK, Kakavandi B, Nasseri S, Mahvi AH, Nabizadeh R, Esrafili A, Kalantary RR (2016) Silica-coated magnetite nanoparticles core-shell spheres (Fe3O4@ SiO2) for natural organic matter removal. J Environ Health Sci Eng 14(1):21
Paulino AT, Belfiore LA, Kubota LT, Muniz EC, Almeida VC, Tambourgi EB (2011) Effect of magnetite on the adsorption behavior of Pb(II), Cd (II), and Cu (II) in chitosan-based hydrogels. Desalination 275(1–3):187–196
Ponomar V, Dudchenko N, Brik A (2018) Synthesis of magnetite powder from the mixture consisting of siderite and hematite iron ores. Miner Eng 122:277–284
Puente-Urbina A, Montero-Campos V (2017) Porous materials modified with Fe3O4 nanoparticles for arsenic removal in drinking water. Water Air ans Soil Pollut 228(9):374
Qin W, Han D, Song X, Engesgaard P (2019) Effects of an abandoned Pb-Zn mine on a karstic groundwater reservoir. J Geochem Explor 200:221–233
Qiufeng Y, Zhao D, Kun Y, Yihong L (2015) Preparation of magnetic Fe3O4 microspheres using different surfactant and silica-coated magnetic particles. Paper presented at the AASRI international conference on industrial electronics and applications (IEA 2015)
Rajput S, Pittman CU Jr, Mohan D (2016) Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J Colloid Interface Sci 468:334–346
Ramachandran A, Prasankumar T, Sivaprakash S, Wiston BR, Biradar S, Jose S (2017) Removal of elevated level of chromium in groundwater by the fabricated PANI/Fe3O4 nanocomposites. Environ Sci Pollut Res 24(8):7490–7498
Ranjbakhsh E, Bordbar A, Abbasi M, Khosropour A, Shams E (2012) Enhancement of stability and catalytic activity of immobilized lipase on silica-coated modified magnetite nanoparticles. Chem Eng J 179:272–276
Razavi N, Es’ haghi Z (2018) Employ of magnetic polyaniline coated chitosan nanocomposite for extraction and determination of phthalate esters in diapers and wipes using gas chromatography. Microchem J 142:359–366
Reddy LH, Arias JL, Nicolas J, Coureur P (2012) Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev 112(11):5818–5878
Ren Y, Abbood HA, He F, Peng H, Huang K (2013) Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption. Chem Eng J 226:300–311
Roberts AP (2015) Magnetic mineral diagenesis. Earth Sci Rev 151:1–47
Rowan CJ, Roberts AP, Broadbent T (2009) Reductive diagenesis, magnetite dissolution, greigite growth and paleomagnetic smoothing in marine sediments: a new view. Earth Planet Sci Lett 277(1–2):223–235
Rufus A, Sreeju N, Philip D (2019) Size tunable biosynthesis and luminescence quenching of nanostructured hematite (α-Fe2O3) for catalytic degradation of organic pollutants. J Phys Chem Solids 124:221–234
Sadeghi MM, Rad AS, Ardjmand M, Mirabi A (2018) Preparation of magnetic nanocomposite based on polyaniline/Fe3O4 towards removal of lead (II) ions from real samples. Synth Met 245:1–9
Santoyo Salazar J, Perez L, De Abril O, Truong Phuoc L, Ihiawakrim D, Vazquez M, Pourroy G (2011) Magnetic iron oxide nanoparticles in 10–40 nm range: composition in terms of magnetite/maghemite ratio and effect on the magnetic properties. Chem Mater 23(6):1379–1386
Sarma L, Sarmah T, Aomoa N, Sarma S, Deshpande U, Bhuyan H, Kakati M (2018) Size-controlled synthesis of superparamagnetic iron-oxide and iron-oxide/iron/carbon nanotube nanocomposites by supersonic plasma expansion technique. J Phys D Appl Phys 51(19):195003
Sayed FN, Polshettiwar V (2015) Facile and sustainable synthesis of shaped iron oxide nanoparticles: effect of iron precursor salts on the shapes of iron oxides. Sci Rep 5:9733
Schmidt A, Nowaczyk N, Kämpf H, Schüller I, Flechsig C, Jahr T (2013) Origin of magnetic anomalies in the large Ebersbrunn diatreme, W Saxony, Germany. Bull Volcanol 75(12):766
Segal I, Zablotskaya A, Lukevics E, Maiorov M, Zablotsky D, Blums E, Gulbe A (2010) Preparation and cytotoxic properties of goethite-based nanoparticles covered with decyldimethyl (dimethylaminoethoxy) silane methiodide. Appl Organomet Chem 24(3):193–197
Shahriman MS, Ramachandran MR, Zain NNM, Mohamad S, Manan NSA, Yaman SM (2018) Polyaniline-dicationic ionic liquid coated with magnetic nanoparticles composite for magnetic solid phase extraction of polycyclic aromatic hydrocarbons in environmental samples. Talanta 178:211–221
Shankar B (2019) A critical assay of heavy metal pollution index for the groundwaters of Peenya Industrial Area, Bangalore, India. Environ Monit Assess 191(5):289
Shapira P, Youtie J (2015) The economic contributions of nanotechnology to green and sustainable growth green processes for nanotechnology. Springer, Berlin, pp 409–434
Shavandi M, Haddadian Z, Ismail M, Abdullah N, Abidin Z (2012) Removal of Fe(III), Mn(II) and Zn (II) from palm oil mill effluent (POME) by natural zeolite. J Taiwan Inst Chem Eng 43(5):750–759
Sikora P, Cendrowski K, Horszczaruk E, Mijowska E (2018) The effects of Fe3O4 and Fe3O4/SiO2 nanoparticles on the mechanical properties of cement mortars exposed to elevated temperatures. Constr Build Mater 182:441–450
Singh M, Ramanathan R, Mayes EL, Mašková S, Svoboda P, Bansal V (2018) One-pot synthesis of maghemite nanocrystals across aqueous and organic solvents for magnetic hyperthermia. Appl Mater Today 12:250–259
Sodipo BK, Aziz AA (2016) Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J Magn Magn Mater 416:275–291
Son YH, Jung Y, Roh H, Lee JK (2017) Enhanced viscoelastic property of iron oxide nanoparticle decorated organoclay fluid under magnetic field. Nano Converg 4(1):22
Song X, Tan L, Ma H, Guo Y, Zhu L, Yi X, Dong Q (2017) Facile preparation of S-doped magnetite hollow spheres for highly efficient sorption of uranium (VI). Dalton Trans 46(10):3347–3352
Song Y, Li Y, Teng Z, Huang Y, Chen X, Wang Q (2018) Size-controlled synthesis of carboxyl-functionalized magnetite particles: effects of molecular weight of the polymer and aging. ACS Omega 3(12):17904–17913
Sui N, Wang L, Wu X, Li X, Sui J, Xiao H, William WY (2015) Polyethylenimine modified magnetic graphene oxide nanocomposites for Cu 2 + removal. RSC Adv 5(1):746–752
Sun Y, Shao D, Chen C, Yang S, Wang X (2013) Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ Sci Technol 47(17):9904–9910 (supercritical condition of ethanol. Journal of Nanomaterials, 2016)
Sun X, Wang S, Wang Y, Sun K (2019) Synthesis and characterization of hydrophobic Fe3O4 magnetic nanoparticles with high saturation magnetization. J Supercond Novel Magnetism. https://doi.org/10.1007/s10948-019-5066-8
Suppiah DD, Johan MR (2018) Influence of solution pH on the formation of iron oxide nanoparticles. Mater Res Express 6(1):015008
Szalai AJ, Manivannan N, Kaptay G (2019) Super-paramagnetic magnetite nanoparticles obtained by different synthesis and separation methods stabilized by biocompatible coatings. Colloids Surf A 568:113–122
Tahar LB, Oueslati MH, Abualreish MJA (2018) Synthesis of magnetite derivatives nanoparticles and their application for the removal of chromium (VI) from aqueous solutions. J Colloid Interface Sci 512:115–126
Tahmasebi E, Yamini Y (2014) Polythiophene-coated Fe3O4 nanoparticles as a selective adsorbent for magnetic solid-phase extraction of silver (I), gold (III), copper (II) and palladium (II). Microchim Acta 181(5–6):543–551
Tahmasebi E, Yamini Y, Mehdinia A, Rouhi F (2012) Polyaniline-coated Fe3O4 nanoparticles: an anion exchange magnetic sorbent for solid-phase extraction. J Sep Sci 35(17):2256–2265
Tamasi G, Cini R (2004) Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci Total Environ 327(1–3):41–51
Tayyebi A, Outokesh M, Moradi S, Doram A (2015) Synthesis and characterization of ultrasound assisted “graphene oxide–magnetite” hybrid, and investigation of its adsorption properties for Sr (II) and Co (II) ions. Appl Surf Sci 353:350–362
Thomé A, Reddy KR, Reginatto C, Cecchin I (2015) Review of nanotechnology for soil and groundwater remediation: Brazilian perspectives. Water Air Soil Pollut 226(4):121
Trpkov D, Panjan M, Kopanja L, Tadić M (2018) Hydrothermal synthesis, morphology, magnetic properties and self-assembly of hierarchical α-Fe2O3 (hematite) mushroom-, cube-and sphere-like superstructures. Appl Surf Sci 457:427–438
Vedavyasan CV (2001) Pontential use of magnetic fields—a perspective. Desalination 134(1–3):105–108
Vistuba JP, Nagel-Hassemer ME, Lapolli FR, Lobo Recio MÁ (2013) Simultaneous adsorption of iron and manganese from aqueous solutions employing an adsorbent coal. Environ Technol 34(2):275–282
Wan C, Li J (2015) Facile synthesis of well-dispersed superparamagnetic γ-Fe2O3 nanoparticles encapsulated in three-dimensional architectures of cellulose aerogels and their applications for Cr(VI) removal from contaminated water. ACS Sustain Chem Eng 3(9):2142–2152
Wang J (2019) Short-term geochemical investigation and assessment of dissolved elements from simulated ash reclaimed soil into groundwater. Environ Pollut 247:302–311
Wang J, Xu W, Chen L, Huang X, Liu J (2014) Preparation and evaluation of magnetic nanoparticles impregnated chitosan beads for arsenic removal from water. Chem Eng J 251:25–34
Wang S, Wang K, Dai C, Shi H, Li J (2015) Adsorption of Pb2 + on amino-functionalized core–shell magnetic mesoporous SBA-15 silica composite. Chem Eng J 262:897–903
Wang Y, Zhang Y, Hou C, He X, Liu M (2016) Preparation of a novel TETA functionalized magnetic PGMA nano-absorbent by ATRP method and used for highly effective adsorption of Hg(II). J Taiwan Inst Chem Eng 58:283–289
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ Geochem Health 36(1):169–182
Wu C (2008) Bayan Obo controversy: carbonatites versus iron oxide–Cu–Au-(REE-U). Resour Geol 58(4):348–354
Wu X, Tan X, Yang S, Wen T, Guo H, Wang X, Xu A (2013) Coexistence of adsorption and coagulation processes of both arsenate and NOM from contaminated groundwater by nanocrystallined Mg/Al layered double hydroxides. Water Res 47(12):4159–4168
Xin T, Ma M, Zhang H, Gu J, Wang S, Liu M, Zhang Q (2014) A facile approach for the synthesis of magnetic separable Fe3O4@ TiO2, core–shell nanocomposites as highly recyclable photocatalysts. Appl Surf Sci 288:51–59
Xu P, Zeng GM, Huang DL, Feng CL, Hu S, Zhao MH, Xie GX (2012) Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ 424:1–10
Xue Y, Wu J, Sun J (2012) Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol Lett 214(2):91–98
Yager TR, Bermúdez-Lugo O, Mobbs PM, Newman HR, Taib M, Wallace G, Wilburn D (2010) The mineral industries of Africa. Minerals Yearbook
Yan H, Li H, Tao X, Li K, Yang H, Li A, Cheng R (2014) Rapid removal and separation of iron (II) and manganese (II) from micropolluted water using magnetic graphene oxide. ACS Appl Mater Interfaces 6(12):9871–9880
Yang ZF, Li LY, Hsieh CT, Juang RS, Gandomi YA (2018) Fabrication of magnetic iron oxide@ graphene composites for adsorption of copper ions from aqueous solutions. Mater Chem Phys 219:30–39
Yano JI, Eguchi I (1979) Application of high gradient magnetic separation for water treatment in steel industry. In: Kiu YA (ed) Industrial applications of magnetic separation. Daido Steel Co., Ltd, Nagoya, Japan, New York, NY, pp 134–136
Yao Y, Miao S, Yu S, Ma LP, Sun H, Wang S (2012) Fabrication of Fe3O4/SiO2 core/shell nanoparticles attached to graphene oxide and its use as an adsorbent. J Colloid Interface Sci 379(1):20–26
Ye N, Xie Y, Shi P, Gao T, Ma J (2014) Synthesis of magnetite/graphene oxide/chitosan composite and its application for protein adsorption. Mater Sci Eng C 45:8–14
Yuan P, Liu D, Fan M, Yang D, Zhu R, Ge F, He H (2010) Removal of hexavalent chromium [Cr(VI)] from aqueous solutions by the diatomite-supported/unsupported magnetite nanoparticles. J Hazard Mater 173(1–3):614–621
Yusoff A, Salimi M, Jamlos M (2018) Critical parametric study on final size of magnetite nanoparticles. In: Paper presented at the IOP conference series: materials science and engineering
Zaaba N, Foo K, Hashim U, Tan S, Liu WW, Voon C (2017) Synthesis of graphene oxide using modified hummers method: solvent influence. Proc Eng 184:469–477
Zawrah MF, El Shereefy ESE, Khudir AY (2018) Reverse precipitation synthesis of ≤ 10 nm magnetite nanoparticles and their application for removal of heavy metals from water. Silicon 11(1):85–104
Zhang H (2016) Magnetic separation nanotechnology for wastewater treatment and used nuclear fuel recycle: University of Idaho
Zhang C, Sui J, Li J, Tang Y, Cai W (2012) Efficient removal of heavy metal ions by thiol-functionalized superparamagnetic carbon nanotubes. Chem Eng J 210:45–52
Zhang J, Zhai S, Li S, Xiao Z, Song Y, An Q, Tian G (2013a) Pb (II) removal of Fe3O4@ SiO2–NH2 core–shell nanomaterials prepared via a controllable sol–gel process. Chem Eng J 215:461–471
Zhang S, Zeng M, Xu W, Li J, Li J, Xu J, Wang X (2013b) Polyaniline nanorods dotted on graphene oxide nanosheets as a novel super adsorbent for Cr(VI). Dalton Trans 42(22):7854–7858
Zhang W, Shi X, Zhang Y, Gu W, Li B, Xian Y (2013c) Synthesis of water-soluble magnetic graphene nanocomposites for recyclable removal of heavy metal ions. J Mater Chem A 1(5):1745–1753
Zhang H, Huang F, Liu DL, Shi P (2015) Highly efficient removal of Cr(VI) from wastewater via adsorption with novel magnetic Fe3O4@ C@ MgAl-layered double-hydroxide. Chin Chem Lett 26(9):1137–1143
Zhao WW, Zhou MF (2015) In-situ LA–ICP-MS trace elemental analyses of magnetite: the mesozoic tengtie skarn Fe deposit in the Nanling Range, south China. Ore Geol Rev 65:872–883
Zhao F, Zhang B, Feng L (2012) Preparation and magnetic properties of magnetite nanoparticles. Mater Lett 68:112–114
Zhao D, Zhang Q, Xuan H, Chen Y, Zhang K, Feng S, Chen C (2017) EDTA functionalized Fe3O4/graphene oxide for efficient removal of U (VI) from aqueous solutions. J Colloid Interface Sci 506:300–307
Zhou L, Deng H, Wan J, Shi J, Su T (2013) A solvothermal method to produce RGO–Fe3O4 hybrid composite for fast chromium removal from aqueous solution. Appl Surf Sci 283:1024–1031
Zhou Q, Li J, Wang M, Zhao D (2016) Iron-based magnetic nanomaterials and their environmental applications. Crit Rev Environ Sci Technol 46(8):783–826
Zhu K, Duan Y, Wang F, Gao P, Jia H, Ma C, Wang C (2017) Silane-modified halloysite/Fe3O4 nanocomposites: simultaneous removal of Cr(VI) and Sb (V) and positive effects of Cr(VI) on Sb (V) adsorption. Chem Eng J 311:236–246
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Usman, U.A., Yusoff, I., Raoov, M. et al. The economic potential of the African iron-ore tailings: synthesis of magnetite for the removal of trace metals in groundwater—a review. Environ Earth Sci 78, 615 (2019). https://doi.org/10.1007/s12665-019-8589-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8589-1