Abstract
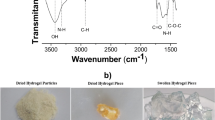
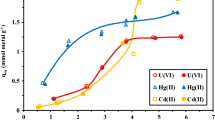
Mine residue and leachate were sampled from an acid mine drainage site near Arroyo San Pedro, which is one of the oldest abandoned mine districts in San Luis Potosi, Mexico, and characterized by X-ray diffraction and inductively coupled plasma-optical emission spectroscopy, confirming the presence of Fe, As, and SO4 2−. To address this problem, chitosan network (net-CS) and chitosan network-N-vinylcaprolactam/N–N-dimethylacrylamide (net-CS)-g-NVCL/DMAAm hydrogels were synthesized and used as adsorbents of the different ions present in the aforementioned leachate by batch equilibrium procedure. Kinetics, isotherms, and ions dissolved in leachate were evaluated. The gels showed the highest adsorption capacity for As and Fe ions. The adsorption capacity of the net-CS hydrogels for As (V) and Fe(III) was 0.786 and 76.85 mg/g, respectively, attained after 50 h. The surface of the hydrogels was investigated by scanning electron microscopy and Fourier transform infrared spectroscopy, before and after the adsorption process, where the presence of a bond between the hydrogels and heavy metals ions, which is commonly observed in organic groups, was observed. In addition, Freundlich and Langmuir adsorption isotherms constants were determined for the As and Fe ions, and it was found that the Freundlich isotherm, with a first-order pseudo model, better fitted the adsorption process, indicating heterogeneous sorption, and the retention process occurred by chemisorption. The results from the Geochemist´s Workbench (GWB) software program revealed that arsenates, such as H3AsO4, H2AsO4 −, as well as Fe++, FeSO4(aq) and FeOH+ were the common aqueous species found in the leachate at pH = 2.9.








Similar content being viewed by others
References
Barakat MA, Sahiner N (2008) Cationic hydrogels for toxic arsenate removal from aqueous environment. J Environ Manag 88:955–961
Barrera-Díaz C, Ramirez E, Burillo G, Roa G, Bilyeu B (2011) Use of pH-sensitive polymer hydrogels in lead removal from aqueous solution. J Hazard Mater 192:432–439
Bethke CM (2008) Geochemical and biogeochemical reaction modeling. Cambridge University Press, Cambridge
Burillo G, González-Gómez R, Ortega A, Lazo LM (2014) Retention of heavy metal ions on comb-type hydrogels based on acrylic acid and 4-vinylpyridine, synthesized by gamma radiation. Radiat Phys Chem 102:117–123
Corrales-Pérez D, Romero FM (2013) Evaluación de la peligrosidad de jales de zonas mineras de Nicaragua y México y alternativas de solución. Bol Soc Geol Mex 65(3):427–446
Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157(2–3):220–229
Essington ME (2005) Soil and water chemistry an integrative approach. CRC Press, Florida
Giménez J, Martínez M, de Pablo J, Rovira M, Duro L (2007) Arsenic sorption onto natural hematite, magnetite and goethite. J Hazard Mater 141:575–580
Hering J, Wilkie J (2003) Adsorption of arsenic onto hydrous ferric oxide: effects of adsorbate/adsorbent ratios and co-occurring solutes. Colloids Surf A 107:97–110
Lee MK, Saunders JA, Harrington J, Lutes C, Wilkin R (2004) Strategy for in situ bioremediation of arsenic in groundwater: field and modeling studies. In Gavaskar AR and Chen ASC (eds.), Battelle Press, Monterey CA, 4C-06
Martínez OA (2015) Movilidad de metales y metaloides en sitios mineros: Predicción e impacto en recursos hídricos. Dissertation. Universidad Autónoma de San Luis Potosi
Masindi V, Gitari MW, Tutu H, DeBeer M (2015) Synthesis of cryptocrystalline magnesite-bentonite clay composite and its application for neutralization and attenuation of inorganic contaminants in acidic and metalliferous mine drainage. J Water Process Eng 11(007):2214–7144
Medellín-Castillo NA, Leyva-Ramos R, Ocampo-Pérez R, García de la Cruz A, Aragón-Pin A, Martinez-Rosales JM, Guerrero-Coronado RM, Fuentes-Rubio L (2007) Adsorption of fluoride from water solution on bone bar. Ind Eng Chem Res 46:9205–9212
Mende M, Schwarz D, Steinbach C, Boldt R, Schwarz S (2016) Simultaneous adsorption of heavy metal ions and anions from aqueous solutions on chitosan − Investigated by spectrophotometry and SEM-EDX analysis. Colloids Surf A. doi:10.1016/j.colsurfa.2016.08.033
Montes JA, Ortega A, Burillo G (2015) Dual-stimuli responsive copolymers based on N-vinylcaprolactam/chitosan. J Radioanal Nucl Chem 303:2143–2150
Nordstrom DK (2011) Hydrogeochemical process governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to Surface waters. Appl Geochem 26:1777–1791
Orozco-Guareño E, Hernández SL, Gómez-Salazar S, Mendizábal E, Katime I (2011) Estudio del hinchamiento de hidrogeles acrílicos terpoliméricos en agua y en soluciones acuosas de ion plumboso. Rev Mex Ing Quim 10(3):465–470
Otero-Fariña A, Gago R, Antelo J, Fiol S, Arce F (2015) Surface complexation modelling of arsenic and copper immobilization by iron oxide precipitates derived from acid mine drainage. Bol Soc Geol Mex 67(3):493–508
Pandey ND, Singh A, Narvi SS, Dutta PK (2006) External stimuli response on a novel chitosan hydrogel crosslinked with formaldehyde. Bull Mater Sci 29:233–238
Pérez-Calixto MP, Ortega A, García-Uriostegui L, Burillo G (2016) Synthesis and characterization of N-vinylcaprolactam/N, N-dimethylacrylamide grafted onto chitosan networks by gamma radiation. Radiat Phys Chem 119:228–235
Rojas B, Ramírez M, Prin JL, Torres C, Bejarano L, Villaroel H, Rojas L, Murillo M, Katime I (2010) Hidrogeles de acrilamida: estudio de su capacidad de remoción de efluentes industriales. Rev Latin Am Metal Mater 30(1):28–39
Saunders JA, Lee MK, Shamsudduha M, Dhakal P, Uddin A, Chowdury MT, Ahmed KM (2008) Geochemistry and mineralogy of arsenic in (natural) anaerobic groundwaters. Appl Geochem 23:3205–3214
Serrano-Gómez J, Burillo G, Bonifacio-Martinez J (2013) Adsorption of Chromium (VI) on Radiation Grafted N, N-dimethylaminoethylmethacrylate onto Polypropylene, from Aqueous Solutions. J Mex Chem Soc 57(2):80–84
Sherman D, Randall S, Ragnarsdottir K (2001) Sorption of As(V) on green rust and lepidocrocite: surface complexes from EXAFS spectroscopy. Geochim Cosmochim Acta 65(7):1015–1023
Strasdeit H, Becker T, Schlaak M (2000) Adsorption of nickel (II), zinc (II), and cadmium (II) by new chitosan derivates. React Funct Polym 44:289–298
Tonkin JW, Balistrieri LS, Murray JW (2002) Modeling metal removal onto natural particles formed during mixing of acid rock drainage with ambient surface water. Environ Sci Technol 36:484–492
Varma AJ, Deshpande SV, Kennedy JF (2003) Metal complexation by chitosan and its derivates: a review. Carbohydr Polym 55:77–93
Acknowledgements
The authors thank to the Consejo Nacional de Ciencia y Tecnología-Fondo Institucional de Fomento Regional para el desarrollo científico, tecnológico y de innovación. (CONACYT-Fordecyt) project 190966 for its financial support, as well as M. Martinez and R. Tovar from the Institute of Metallurgy UASLP and H. Davila from (Chemistry), S. Mendoza (Environmental), E. Espinoza, M. E. Garcia (Geology) and I. Montes (Engineering) Faculty at UASLP for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burillo, J.C., Castro-Larragoitia, J., Burillo, G. et al. Removal of arsenic and iron from mine-tailing leachate using chitosan hydrogels synthesized by gamma radiation. Environ Earth Sci 76, 450 (2017). https://doi.org/10.1007/s12665-017-6780-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6780-9