Abstract
In the present study, sediments and biotas from two freshwater lakes in Yangze delta area were collected and analyzed for polybrominated diphenyl ethers (PBDEs) and methoxylated PBDEs (MeO–PBDEs). The concentrations of PBDEs in sediments and biotas ranged from 0.41 to 5.8 ng/g dry weight and from 4.6 to 100 ng/g lipid weight, respectively, while those of MeO–PBDEs were much lower (sediment: <LOQ-0.014 ng/g dry weight, biota: <LOQ-2.1 ng/g lipid weight). The levels of both brominated substances in sediments and biotas were in the moderate to low range compared with other studies. Different BDE congeners were found between two lakes probably due to the different exogenous sources and metabolic stages. Similar occurrence of higher brominated congeners (e.g., BDE-209) in sediments and biotas indicated sediments as a possible source of PBDEs for biotas. The different contribution of lower and higher brominated congeners between sediments and biotas may be due to the combined effect of biotransformation and bioavailability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polybrominated diphenyl ethers (PBDEs) have been widely used as additive flame retardant in the textile and plastic products in order to prevent the accident fire (de Wit 2002). Since there are no chemical bounds between additives and products, PBDEs can gradually leach into the environment during the whole life cycle of products (Su et al. 2014). As a result, increasing environmental levels of PBDEs have been reported in various environmental media such as soil (Hale et al. 2006; Luo et al. 2009), sediment (Bradley et al. 2011), wildlife (Law et al. 2007; Muir et al. 2006) and humans (Frederiksen et al. 2009; Haraguchi et al. 2016). Besides, many investigations have shown that some PBDEs have bioaccumulation and biomagnification effects in several aquatic ecosystems (Boon et al. 2002; Hu et al. 2010b; Kelly et al. 2008a; Law et al. 2007) and can undergo long-range transport (LRT) (Bradley et al. 2011) (Frank and Dugani 2003; Law et al. 2006). Several toxicological investigations have been done indicating that PBDEs can cause thyroid hormone disruption (Richardson et al. 2008), reproductive effects (Van den Steen et al. 2009) and neurotoxicity (Costa et al. 2014). Due to their lipophilic characteristics, ubiquity in the environment as well as the adverse effect to biotas, penta-BDE and octa-BDE formulations were listed in the Stockholm Convention on Persistent Organic Pollutants (POPs) in May 2009.
In recent years, great interests have been shown in the study of methoxylated PBDEs (MeO–PBDEs) which are structurally related to PBDEs. There is no commercial use of MeO–PBDEs, and no report indicates it as by-product in industrial processes (Teuten et al. 2005). MeO–PBDEs have been widely detected in wildlife and humans (Bradley et al. 2011; Lacorte and Ikonomou 2009; Qiu et al. 2012; Su et al. 2010). The bulk of studies done mainly focused on marine organisms (Baron et al. 2013; Kelly et al. 2008b; Losada et al. 2009; Malmvärn et al. 2005; Zhang et al. 2010a). Relatively high concentrations of MeO–PBDEs were found in marine algae (Malmvärn et al. 2008) and sponge (Haraguchi et al. 2011) compared with the concentrations of PBDEs, suggesting the possible natural origins. However, little research has been conducted on MeO–PBDE levels of freshwater habitats.
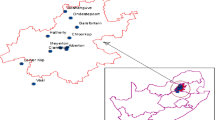
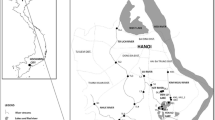
Tai Lake and Dianshan Lake lie in silting alluviation plain of the Yangtze River delta (Fig. 1), which is the economy-developed region and has a large number of manufacturers of chemicals, textile and electronics in China. Tai Lake, located in the boundary of Jiangsu and Zhejiang provinces, is the third largest freshwater lake in China with an area of 2338 km2, while Dianshan Lake is the largest freshwater lake in Shanghai. Both the freshwater lakes function as supplier for drinking water and habitats for aquatic products in the surrounding area. Recently, these two lakes suffered from eutrophication (Le et al. 2010) and water pollution problems including POPs (Qiu et al. 2012; Yu et al. 2012) due to the pressure of huge population, urbanization and industrialization in Yangtze delta area.
The purpose of the present study is to investigate the levels of PBDEs and its structural analogues MeO–PBDEs in the sediments and some freshwater organisms from Tai Lake and Dianshan Lake. The differences in contaminant levels and congener distribution were compared between two lakes. Possible transfer from sediments to biota and biotransformation of PBDEs were also discussed.
Materials and methods
Sampling
In November 2013, three fish species including crucian carp (Carassius auratus, n = 10), silver carp (Hypophthalmichthys molitrix, n = 5) and common carp (Cyprinus carpio, n = 10), and one species of pond snail (Bellamya aeruginosa, 8 pools) were collected from Tai Lake and Dianshan Lake. Surface sediment samples were collected simultaneously from 18 sites (Fig. 2) in Tai Lake and 9 sites in Dianshan Lake by using sediment core sampler. The sediment samples collected from Dianshan Lake were mixed into one pooled sample. The pond snail samples in Tai Lake were collected from the same location of some sediment sampling sites. The biota samples were covered with aluminum foil, and sediment samples were packed in the stainless steel box upon collection. All samples were stored at −20 °C before analysis. Snail samples from the same site were pooled due to its small individual sizes. Back muscle tissues were taken from fish individuals for analysis. Details regarding the fish species are provided in Table S1.
Chemicals and standards
PBDE standard solutions (including BDE-28, BDE-47, BDE-66, BDE-99, BDE-100, BDE-153, BDE-154, BDE-183, BDE-209) were purchased from Cambridge Isotope Laboratories Inc (Andover, MA USA). Two MeO–PBDEs standards (2′-MeO–BDE-68 and 6-MeO–BDE-47) as well as surrogate and internal standards (BDE-77, BDE-138 and BDE-139) were obtained from AccuStandard Inc (New Haven, CT, USA). All solvents used were of highest commercially available purity. Silica gel (100–200 mesh) was activated over night at 300 °C.
Extraction and cleanup
Different extraction methods were used for the samples collected from two lakes. Both samples were firstly frozen-dried. Then, 2 g samples from Tai Lake were soxhlet extracted using acetone/cyclohexane (150 mL, 1:1) with surrogate standard (BDE-138 5 ng/mL 100 μL) for 48 h at 70 °C (additional activated copper was added for sediment samples to remove sulfur). About 2.5 g samples from Dianshan Lake was mixed with 7.5 g water and surrogate standard BDE-77 (20 ng/mL 100 μL) and homogenized for 1 min in a 100-mL glass centrifuge tube with a mixture of 2-propanol (25 mL) and iso-hexane/diethyl ether (3:1 v/v, 20 mL). After centrifugation (5 min at 2300 rpm), the liquid phase was decanted into a separation funnel. The procedure was repeated with a mixture of 2-propanol (10 mL) and iso-hexane/diethyl ether (3:1 v/v, 40 mL). The combined extracts were washed twice by inverting the funnel 30 times with HCl (0.2 M) in aqueous sodium chloride (0.9%) (v/v, 50 + 20 mL).
Both extracts from two lakes were transferred into a test tube using iso-hexane (4 mL). Concentrated sulfuric acid (4 mL) was added to each sample for lipids removal, gently turned over for 1.5 min and centrifuged for 5 min at 3000 rpm. The organic phase was transferred into a new test tube and concentrated to 0.5 mL using a gentle stream of nitrogen. The procedure was repeated once.
Further clean-up was carried out by using activated silica gel columns. A Pasteur pipette was packed with activated silica gel (0.1 g) and activated silica gel (0.9 g) impregnated with concentrated sulfuric acid (2:1 w/w) from down to top. The columns were washed with iso-hexane/dichloromethane (1:1, 5 mL). Then, the extracts were applied to the column and analytes eluted with iso-hexane/dichloromethane (1:1, 15 mL). After clean-up, the solvent was changed to n-hexane and the internal standard, BDE-139 (5 ng/mL 100 μL), was added prior to GC/MS analysis (final volume of 100 μL).
Instrumental analysis
Analysis of PBDEs and MeO–PBDEs was done by using gas chromatography–mass spectrometry (GC–MS) in selected ion monitoring mode (SIM) (bromide isotopes m/z 79 and 81). Automated 1 μL injection with a CTC GC Pal autosampler was conducted on a Varian 450-GC connected to a Varian 320-MS. A programmable temperature vaporizing (PTV) injector was used with an Agilent J&W DB-5HT (15 m 0.25 mm × 0.1 μm) column. Helium was used as carrier gas at a set constant flow of 1.5 mL/min. Methane was used as reagent gas. The PTV injector was set at 260 °C. The injections were performed in splitless mode for 1 min. The ion source temperature and the transfer line temperature were set to 230 and 300 °C, respectively. The oven was programmed as follows: 55 °C for 2 min, ramp 15 °C/min to 310 °C and hold 5 min.
QA/QC
All glassware were washed and heated at 400 °C for 4 h before use, and no plastic equipment was used in the whole procedures. The recoveries of surrogate standards ranged from 60 to 90% for Tai Lake samples (BDE-138) and from 71 to 104% for Dianshan Lake samples (BDE-77).
For every three samples, one procedural blank was used to avoid interference and cross-contamination. The limit of quantification (LOQ) was calculated as three times the instrumental limit of detection (LOD). LOD was defined as the lowest amount needed for detection at a signal-to-noise ratio (S/N) of 3. More information on LOQ is given in Table S2. All biota results were presented as ng/g lipid weight (lw), while sediment samples were calculated as ng/g dry weight (dw).
Statistical analysis
The statistical analysis of data was carried out by using SPSS Statistics 23. Spearman rank correlation was used to examine the differences of pond snails from different sites. It was considered statistically significant when p < 0.05. The figure of congener profile was performed by using OriginPro 2016.
Results and discussion
Concentration and congener profile in sediment samples
The overall concentrations of PBDEs and MeO–PBDEs in biota and sediment samples are listed in Table 1. Concentrations given on a congener basis can be found in Table S3. Seven out of 9 targeted PBDEs were detected in sediment samples from Tai Lake. Three less brominated congeners, BDE-28, BDE-47 and BDE-66, were not found in all sediment samples from Tai Lake. ∑PBDEs (sum of BDE-28, BDE-47, BDE-66, BDE-99, BDE-100, BDE-153, BDE-154, BDE-183 and BDE-209) concentrations of sediment samples from Tai Lake varied from 0.41 to 2.5 ng/g dw, while that of pooled sample from Dianshan Lake was 5.8 ng/g dw.
The total concentration of PBDEs in surficial sediment from Tai Lake and Dianshan Lake is much lower compared to other studies around the world (Table S4). The spatial distribution of ∑8PBDEs and BDE-209 in surficial sediment from Tai Lake (this study) is described in Fig. 2. The highest ∑8PBDEs and BDE-209 concentrations were found at site 10 (1.4 ng/g dw) and site 8 (1.3 ng/g dw), respectively. Interestingly, sediment collected from northeast of Tai Lake (site 1, 2, 16, 18), which are near two big cities Wuxi and Suzhou, showed relatively low PBDE levels (BDE-209: 0.081–0.30 ng/g dw). In recent years, due to the drinking water crisis caused by heavy pollution and eutrophication in Tai Lake, actions like desilting have been taken by local government (Le et al. 2010). We speculated the low concentration of PBDEs in surficial sediment from Tai Lake observed in this study might be related to the removal of sediment. Zhou et al. (2012) reported that BDE-209 and ∑8PBDEs concentrations in sediment collected from Tai Lake in 2010 ranged from 0.13 to 32.62 ng/g dw and from 9.68 to 144 ng/g dw, respectively, which was one to two order of magnitude higher than this study, also supported this speculation. Higher level of PBDEs was found in pooled sediment sample from Dianshan Lake (BDE-209: 5.80 ng/g dw, ∑8PBDE: 0.0207 ng/g dw).
The congener profile of PBDEs in sediment samples from Tai Lake and Dianshan Lake is shown in Fig. 3. In sediments collected from Tai Lake, BDE-209 and BDE-153 were the two most abundant congeners accounting for 46 and 44%, respectively, followed by BDE-154. For sediment from Dianshan Lake, BDE-209 was the major congener consisting of over 99%. The great proportions of deca-BDE were also observed in other studies (Hu et al. 2010a; Mariani et al. 2008; Wang et al. 2015). The predominance of BDE-209 indicated the wide usage of deca-BDE in the study area. Two hexa-BDE congeners BDE-153 and BDE-154 accounted for 44 and 6.6% in the sediments from Tai Lake. Since these two congeners were found in the technical penta-BDE and octa-BDE mixtures (La Guardia et al. 2006), the penta- and octa- technical products could be the original sources for hexa-BDEs detected in the sediment from Tai Lake.
Compared with PBDEs, ∑MeO–PBDEs (sum of 2′-MeO–BDE-68 and 6-MeO–BDE-47) concentrations in sediments from Tai Lake were 100–200 folds lower, ranging from <LOQ (below limit of quantification) to 0.014 ng/g dw. All concentrations of 6-MeO–BDE-47 were below LOQ in sediment samples. Fewer researchers paid attention to the levels of MeO–PBDEs in sediments. Compared with the limited studies, the MeO–PBDEs level in Tai Lake sediment (nd-12 pg/g dw) was similar to that of Liaodong Bay, North China (3.8–56 pg/g dw) (Zhang et al. 2012) but less than that of Muskegon Lake, Michigan, USA (3.5–120 pg/g dw) (Bradley et al. 2011).
Concentration and congener profile in biota samples
All target compounds including 9 PBDE congeners were detected in all aquatic organisms. For all biota from Tai Lake, the concentrations of ∑PBDEs ranged from 4.6 to 100 ng/g lw with mean value of 21 ng/g lw. Pond snail has higher concentration of PBDEs than other biotas from Tai Lake (mean: 31 ng/g lw, range: 6.2–100 ng/g lw). For aquatic organisms from Dianshan Lake, relatively higher concentration of ∑PBDEs was found in common carp (12 ng/g lw) whereas the lowest was detected in pond snail (5.5 ng/g lw).
The comparisons with similar benthic organisms and same fish species in other studies from China are summarized and given in Table S5. In general, the PBDE concentrations in freshwater species in this study were similar to those found in China (Table S5) but less than those reported from other regions and water systems, e.g., Baltic Sea and Canada (Law et al. 2007; Malmvärn et al. 2005; Muir et al. 2006). The PBDE concentrations of pond snail collected from 7 different sites in Tai Lake were in considerably wide range from 6.59 to 114 ng/g lw. The box plot for PBDEs concentrations of pond snails from Tai Lake (Fig. 4) further illustrated the different contaminant levels for bottom-dwelling organisms among different sites. The difference between the western lake and other part of the lake was significant (p < 0.05). Total PBDEs concentrations of pond snails in this study were similar to the levels of crab (Gao et al. 2009), Chinese mystery snail (Wu et al. 2008) and river snail (Hu et al. 2010b) from other freshwater lake ecosystems in China. The PBDEs concentrations in three fish species from Tai Lake and Dianshan Lake were in narrow range, which was probably due to their similar feeding habits (Table S1). Among three fish species, crucian carp seemed to have lower PBDE burdens. The PBDE levels of three fish species in this study were lower than those freshwater fish species from Yangze River and Baiyangdian Lake and one magnitude lower than those from Qingyuan e-waste sites (Luo et al. 2007; Wu et al. 2008), China. Besides, the PBDE results were comparable with data of other freshwater fish species like mandarin fish (Qiu et al. 2012) and anchovy (Su et al. 2010) in Yangze Delta area. In general, the levels of ∑PBDEs in aquatic organisms from Tai Lake were higher than those from Dianshan Lake. However, BDE-47 concentrations of biotas from two lakes were in similar levels.
The congener profiles of PBDEs in four aquatic species are shown in Fig. 3. BDE-153, BDE-209 and BDE-47 were the three most abundant congeners in snail samples from Tai Lake, while for snails from Dianshan Lake BDE-209 and BDE-183 accounted for 44% of PBDEs. Relatively high proportions of BDE-183 and BDE-99 were found in snails from both two lakes compared with fish species. Similar results were also found in shrimp and crab from Baiyangdian Lake (Hu et al. 2010b). In fish, BDE-183 and BDE-99 tend to debrominate to form BDE-154 and BDE-47, respectively (Stapleton et al. 2004; Zeng et al. 2012). According to the observations above, benthic organisms seem to be resistant to debromination and may indicate lower metabolic capacity compared to fish. In fish species collected from Tai Lake, similar distributions were found with BDE-153, BDE-209 and BDE-47, which accounts for 38, 21 and 16%, respectively. BDE-47 contributed the greatest proportion (44%) in fish from Dianshan Lake, followed by BDE-100, BDE-154 and BDE-209. The high proportion of BDE-47 in aquatic species was consistent with previous researches (Law et al. 2007; Su et al. 2014). The metabolism of higher brominated BDEs (e.g., BDE-99) in fish as well as high bioavailability of BDE-47 may explain this result (Gandhi et al. 2006). BDE-153 was also widely detected in various biotas (Hu et al. 2010b; Law et al. 2007; Yogui and Sericano 2009). The dietary exposure test of Juvenile Carp demonstrated the debromination of BDE-209 to hexa- and penta-BDE (Stapleton et al. 2004). The high proportion of BDE-153 in fish from Tai Lake may be attributed to the debromination of higher brominated congeners (e.g., BDE-209). The high detection rates and relatively high concentrations of BDE-209 in fish from two lakes illustrated the bioavailability of BDE-209 in aquatic organisms (Kobayashi et al. 2015; Wu et al. 2008; Yu et al. 2012). The different BDE congeners detected from two lakes might be caused by different metabolic stages of the biotas.
MeO–PBDEs were merely found in pond snails and common carp from Tai Lake with lower levels compared to PBDEs, ranging from <LOQ to 2.1 ng/g lw. For aquatic organisms from Dianshan Lake, all 2′-MeO–BDE-68 concentrations were below LOQ. The ∑MeO–PBDE concentrations in biota samples from this study were comparable or less than those from freshwater organisms in China (Qiu et al. 2012; Su et al. 2010; Zhang et al. 2010a). However, in marine ecosystems, considerably higher MeO–PBDEs concentrations were detected in several biotas (Covaci et al. 2008; Kelly et al. 2008b; Losada et al. 2009) (Table S6). Several studies showed MeO–PBDE levels can be much higher than PBDEs suggesting MeO–PBDEs had a natural origin in marine ecosystems (Haraguchi et al. 2011; Malmvärn et al. 2008; Teuten et al. 2005). In this study, MeO–PBDEs concentrations in biotas were 4–90 times less than PBDEs. Similar relationship of these two compounds was also found in Chinese sturgeon from Yangze River (Zhang et al. 2010b). The MeO–PBDEs in biotas from Tai Lake were at the same low level as those from Dianshan Lake, but different congeners dominated in biotas from two lakes. The ratio of ∑MeO–PBDE to ∑PBDE for biotas in this study ranged from 0.011 to 0.32. Kelly found the ∑PBDE/∑MeO–PBDE ratio was in the range of 0.02–0.6 for cod, sculpin and seaducks in Canadian arctic (Kelly et al. 2008b).
Comparison between biotas and sediments
Since water is a very hydrophilic medium, the solubility of lipophilic compounds like PBDEs is relatively low in water phase. On the contrary, sediment is likely to accumulate lipophilic compounds thus acting as a key compartment as well as a sink of compounds like PBDEs. Shaw attributed the presence of BDE-209 in marine biotas to the ingestion of sediment-associated prey organisms such as zooplankton and benthic invertebrates (Shaw et al. 2009). In the present study, the benthic invertebrates, pond snails from two lakes accounted for high proportions of BDE-153, BDE-154 and BDE-209. Considering the dominance of BDE-209 as well as the presence of BDE-153 and BDE-154 in sediments from two lakes, these congeners detected in pond snails may be derived from sediments. Moreover, in three fish species common carp and crucian carp are benthic fishes which possibly feed on sediment-associated organisms (Table S1). Therefore, the high detection rates and concentrations of BDE-209 in all fish species from two lakes indicated sediment might also be the original source of some higher brominated congeners in fish.
Higher composition of lower brominated congeners (e.g., BDE-28, BDE-47 and BDE-100) as well as the great proportion of higher brominated ones (e.g., BDE-209 and BDE-183) can be observed in biotas and sediments separately from both two lakes. The different distribution of PBDEs among sediments, pond snails and three fish species indicated different environmental processes happened in abiotic environment and biotas. Besides simple partitioning, biotransformation and bioavailability are two key factors for biotas to accumulate the organic pollutants from abiotic compartment. Dietary exposure studies have already shown that higher brominated congeners tended to debrominate into lower brominated ones in fish (e.g., BDE-99 to BDE-47) (Stapleton et al. 2004), thus making BDE-47 and BDE-153 dominant congeners in biotas. On the other side, congeners with large molecules (e.g., BDE-209) usually have a low uptake rate which impede their transport across cell membranes (Ciparis and Hale 2005) resulting in poor bioaccumulation in vivo.
Conclusions
The levels and congener profiles of PBDEs and MeO–PBDEs in sediments and biotas from two freshwater lakes were reported. The selected fish species showed close PBDE concentrations range. The levels of anthropogenic and natural brominated substances in sediments and biotas were in the moderate to low range compared with other studies. The different compositions of BDE congeners found from sediment and biotas in two lakes may be due to the different exogenous sources and metabolic stages. Similar occurrence of higher brominated congeners in biotas and sediments from the same lake indicated sediments as a possible source of PBDEs for biotas. The different distribution of lower and higher brominated congeners between sediments and biotas may be attributed to the combined effect of biotransformation and bioavailability.
Change history
02 August 2019
The article “Polybrominated diphenyl ethers and its methoxylated analogues in biota and sediment samples from two freshwater lakes in Yangtze River delta, written by Xinyu Du, Hong Chang, Yihui Zhou, Yanling Qiu, Yan Wu, Zhifen Lin, Zhiliang Zhu and Jianfu Zhao, was originally published
References
Baron E, Rudolph I, Chiang G, Barra R, Eljarrat E, Barcelo D (2013) Occurrence and behavior of natural and anthropogenic (emerging and historical) halogenated compounds in marine biota from the Coast of Concepcion (Chile). Sci Total Environ 461–462:258–264. doi:10.1016/j.scitotenv.2013.05.006
Boon JP et al (2002) Levels of polybrominated diphenyl ether (PBDE) flame retardants in animals representing different trophic levels of the North Sea food web. Environ Sci Technol 36:4025–4032. doi:10.1021/es0158298
Bradley PW et al (2011) PBDEs and methoxylated analogues in sediment cores from two Michigan, USA, inland Lakes. Environ Toxicol Chem 30:1236–1242. doi:10.1002/etc.500
Ciparis S, Hale RC (2005) Bioavailability of polybrominated diphenyl ether flame retardants in biosolids and spiked sediment to the aquatic oligochaete, Lumbriculus variegatus. Environ Toxicol Chem 24:916–925. doi:10.1897/04-179r.1
Costa LG, de Laat R, Tagliaferri S, Pellacani C (2014) A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett 230:282–294. doi:10.1016/j.toxlet.2013.11.011
Covaci A et al (2008) Anthropogenic and naturally occurring organobrominated compounds in two deep-sea fish species from the Mediterranean Sea. Environ Sci Technol 42:8654–8660. doi:10.1021/es8016528
de Wit CA (2002) An overview of brominated flame retardants in the environment. Chemosphere 46:583–624. doi:10.1016/s0045-6535(01)00225-9
Frank W, Dugani CB (2003) Assessing the long-range transport potential of polybrominated diphenyl ethers: a comparison of four multimedia models. Environ Toxicol Chem 22:1252–1261
Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE (2009) Human internal and external exposure to PBDEs—a review of levels and sources. Int J Hyg Environ Health 212:109–134. doi:10.1016/j.ijheh.2008.04.005
Gandhi N, Bhavsar SP, Gewurtz SB, Diamond ML, Evenset A, Christensen GN, Gregor D (2006) Development of a multichemical food web model: application to PBDEs in Lake Ellasjøen, Bear Island, Norway. Environ Sci Technol 40:4714–4721. doi:10.1021/es052064l
Gao Z, Xu J, Xian Q, Feng J, Chen X, Yu H (2009) Polybrominated diphenyl ethers (PBDEs) in aquatic biota from the lower reach of the Yangtze River, East China. Chemosphere 75:1273–1279. doi:10.1016/j.chemosphere.2009.01.065
Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor TM (2006) Brominated flame retardant concentrations and trends in abiotic media. Chemosphere 64:181–186. doi:10.1016/j.chemosphere.2005.12.006
Haraguchi K, Kato Y, Ohta C, Koga N, Endo T (2011) Marine sponge: a potential source for methoxylated polybrominated diphenyl ethers in the Asia-Pacific food web. J Agric Food Chem 59:13102–13109. doi:10.1021/jf203458r
Haraguchi K, Ito Y, Takagi M, Fujii Y, Harada KH, Koizumi A (2016) Levels, profiles and dietary sources of hydroxylated PCBs and hydroxylated and methoxylated PBDEs in Japanese women serum samples. Environ Int 97:155–162
Hu G et al (2010a) Distribution of polybrominated diphenyl ethers and decabromodiphenylethane in surface sediments from Fuhe River and Baiyangdian Lake. North China J Environ Sci (China) 22:1833–1839
Hu GC et al (2010b) Bioaccumulation behavior of polybrominated diphenyl ethers (PBDEs) in the freshwater food chain of Baiyangdian Lake, North China. Environ Int 36:309–315. doi:10.1016/j.envint.2010.01.002
Kelly BC, Ikonomou MG, Blair JD, Gobas FA (2008a) Bioaccumulation behaviour of polybrominated diphenyl ethers (PBDEs) in a Canadian Arctic marine food web. Sci Total Environ 401:60–72. doi:10.1016/j.scitotenv.2008.03.045
Kelly BC, Ikonomou MG, Blair JD, Gobas FA (2008b) Hydroxylated and methoxylated polybrominated diphenyl ethers in a Canadian Arctic marine food web. Environ Sci Technol 42:7069–7077. doi:10.1021/es801275d
Kobayashi J et al (2015) Trophic magnification of polychlorinated biphenyls and polybrominated diphenyl ethers in an estuarine food web of the Ariake Sea. Jpn Chemosphere 118:201–206. doi:10.1016/j.chemosphere.2014.08.066
La Guardia MJ, Hale RC, Harvey E (2006) Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta- octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol 40:6247–6254. doi:10.1021/es060630m
Lacorte S, Ikonomou MG (2009) Occurrence and congener specific profiles of polybrominated diphenyl ethers and their hydroxylated and methoxylated derivatives in breast milk from Catalonia. Chemosphere 74:412–420. doi:10.1016/j.chemosphere.2008.09.050
Law RJ et al (2006) Levels and trends of brominated flame retardants in the European environment. Chemosphere 64:187–208. doi:10.1016/j.chemosphere.2005.12.007
Law K et al (2007) Bioaccumulation and trophic transfer of some brominated flame retardants in a Lake Winnipeg (Canada) food web. Environ Toxicol Chem 25:2177–2186
Le C, Zha Y, Li Y, Sun D, Lu H, Yin B (2010) Eutrophication of lake waters in China: cost, causes, and control. Environ Manage 45:662–668. doi:10.1007/s00267-010-9440-3
Losada S et al (2009) Biomagnification of anthropogenic and naturally-produced organobrominated compounds in a marine food web from Sydney Harbour, Australia. Environ Int 35:1142–1149. doi:10.1016/j.envint.2009.07.008
Luo Q, Wong M, Cai Z (2007) Determination of polybrominated diphenyl ethers in freshwater fishes from a river polluted by e-wastes. Talanta 72:1644–1649. doi:10.1016/j.talanta.2007.03.012
Luo Y, Luo XJ, Lin Z, Chen SJ, Liu J, Mai BX, Yang ZY (2009) Polybrominated diphenyl ethers in road and farmland soils from an e-waste recycling region in Southern China: concentrations, source profiles, and potential dispersion and deposition. Sci Total Environ 407:1105–1113. doi:10.1016/j.scitotenv.2008.10.044
Malmvärn A, Zebuhr Y, Kautsky L, Bergman K, Asplund L (2008) Hydroxylated and methoxylated polybrominated diphenyl ethers and polybrominated dibenzo-p-dioxins in red alga and cyanobacteria living in the Baltic Sea. Chemosphere 72:910–916. doi:10.1016/j.chemosphere.2008.03.036
Malmvärn A, Marsh G, Kautsky L, Athanasiadou M, Bergman Å, Asplund L (2005) Hydroxylated and methoxylated brominated diphenyl ethers in the red algaeceramium tenuicorneand blue mussels from the Baltic Sea. Environ Sci Technol 39:2990–2997. doi:10.1021/es0482886
Mariani G et al (2008) Atmospheric input of POPs into Lake Maggiore (Northern Italy): PBDE concentrations and profile in air, precipitation, settling material and sediments. Chemosphere 73:S114–S121. doi:10.1016/j.chemosphere.2007.02.071
Muir DC et al (2006) Brominated flame retardants in polar bears (Ursus maritimus) from Alaska, the Canadian Arctic, East Greenland, and Svalbard. Environ Sci Technol 40:449–455. doi:10.1021/es051707u
Qiu Y et al (2012) Chlorinated and brominated organic contaminants in fish from Shanghai markets: a case study of human exposure. Chemosphere 89:458–466. doi:10.1016/j.chemosphere.2012.05.099
Richardson VM, Staskal DF, Ross DG, Diliberto JJ, DeVito MJ, Birnbaum LS (2008) Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharmacol 226:244–250. doi:10.1016/j.taap.2007.09.015
Shaw SD, Berger ML, Brenner D, Kannan K, Lohmann N, Papke O (2009) Bioaccumulation of polybrominated diphenyl ethers and hexabromocyclododecane in the northwest Atlantic marine food web. Sci Total Environ 407:3323–3329. doi:10.1016/j.scitotenv.2009.02.018
Stapleton HM, Alaee M, Letcher RJ, Baker JE (2004) Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environ Sci Technol 38:112–119. doi:10.1021/es034746j
Su GY et al (2010) Polybrominated diphenyl ethers and their methoxylated metabolites in anchovy (Coilia sp.) from the Yangtze River delta, China. Environ Sci Pollut Res Int 17:634–642. doi:10.1007/s11356-009-0236-z
Su G, Saunders D, Yu Y, Yu H, Zhang X, Liu H, Giesy JP (2014) Occurrence of additive brominated flame retardants in aquatic organisms from Tai Lake and Yangtze River in Eastern China, 2009–2012. Chemosphere 114:340–346. doi:10.1016/j.chemosphere.2014.05.046
Teuten EL, Xu L, Reddy CM (2005) Two abundant bioaccumulated halogenated compounds are natural products. Science 307:917–920. doi:10.1126/science.1106882
Van den Steen E, Eens M, Covaci A, Dirtu AC, Jaspers VL, Neels H, Pinxten R (2009) An exposure study with polybrominated diphenyl ethers (PBDEs) in female European starlings (Sturnus vulgaris): toxicokinetics and reproductive effects. Environ Pollut 157:430–436. doi:10.1016/j.envpol.2008.09.031
Wang XT et al (2015) Occurrence, profiles, and ecological risks of polybrominated diphenyl ethers (PBDEs) in river sediments of Shanghai, China. Chemosphere 133:22–30. doi:10.1016/j.chemosphere.2015.02.064
Wu JP, Luo XJ, Zhang Y, Luo Y, Chen SJ, Mai BX, Yang ZY (2008) Bioaccumulation of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in wild aquatic species from an electronic waste (e-waste) recycling site in South China. Environ Int 34:1109–1113. doi:10.1016/j.envint.2008.04.001
Yogui GT, Sericano JL (2009) Polybrominated diphenyl ether flame retardants in the U.S. marine environment: a review. Environ Int 35:655–666. doi:10.1016/j.envint.2008.11.001
Yu YX et al (2012) Polybrominated diphenyl ethers and polychlorinated biphenyls in freshwater fish from Taihu Lake, China: their levels and the factors that influence biomagnification. Environ Toxicol Chem 31:542–549. doi:10.1002/etc.1722
Zeng YH, Luo XJ, Chen HS, Yu LH, Chen SJ, Mai BX (2012) Gastrointestinal absorption, metabolic debromination, and hydroxylation of three commercial polybrominated diphenyl ether mixtures by common carp. Environ Toxicol Chem 31:731–738. doi:10.1002/etc.1716
Zhang K, Wan Y, An L, Hu J (2010a) Trophodynamics of polybrominated diphenyl ethers and methoxylated polybrominated diphenyl ethers in a marine food web. Environ Toxicol Chem 29:2792–2799. doi:10.1002/etc.334
Zhang K, Wan Y, Giesy JP, Lam MH, Wiseman S, Jones PD, Hu J (2010b) Tissue concentrations of polybrominated compounds in Chinese sturgeon (Acipenser sinensis): origin, hepatic sequestration, and maternal transfer. Environ Sci Technol 44:5781–5786. doi:10.1021/es100348g
Zhang K, Wan Y, Jones PD, Wiseman S, Giesy JP, Hu J (2012) Occurrences and fates of hydroxylated polybrominated diphenyl ethers in marine sediments in relation to trophodynamics. Environ Sci Technol 46:2148–2155. doi:10.1021/es203195s
Zhou P et al (2012) Distribution of polybrominated diphenyl ethers in the surface sediments of the Taihu Lake, China. Chemosphere 88:1375–1382. doi:10.1016/j.chemosphere.2012.05.048
Acknowledgements
The work was supported by Shanghai Science and Technology Commission (14DZ2261100), International Science and Technology Cooperation Program of China (2016YFE0123700) and VR Research Cooperation China project (639-2013-6913).
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised due to a retrospective Open Access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Du, X., Chang, H., Zhou, Y. et al. Polybrominated diphenyl ethers and its methoxylated analogues in biota and sediment samples from two freshwater lakes in Yangtze River delta. Environ Earth Sci 76, 171 (2017). https://doi.org/10.1007/s12665-017-6499-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6499-7