Abstract
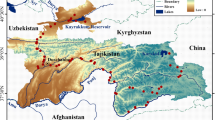
Hydrochemical parameters and stable isotope (18O and 2H) ratios were monitored at 10 stations along the Euphrates River (ER) within the Syrian reach during 2004–2006. The average concentrations of major ions in the ER were comparable to those of semi-arid and arid zone rivers. Temporal variations of major ion concentrations were small at the first two upper sites and more pronounced at the lower downstream stations. Temporal fluctuations of stable isotope ratios at the first two upper sites were identical and have a distinct opposite evolution trend to those of the remaining downstream stations, with depleted values during summer–autumn and enriched values during February–May. Salinity and stable isotope values of the ER systemically increase with the distance downstream, with sharp enrichments in stable isotope values at Al-Assad Lake, primarily because of evaporation from the lake. The chemical and isotopic properties of the ER water suggest negligible role of precipitation and local runoff compared with evaporation. Cluster analysis in Q-mode resulted in classifying the major ions into: the most predominant species (SO4 2−, HCO3 −, Cl−, Ca2+ and Na+), liberated as a result of rock–water interactions or dissolution of soluble evaporate salts, and the less abundant ions (Mg2+, K+, NO3 − and SiO2), partially imported from different sources (rainfall, silicate weathering and anthropogenic pollution). Factor analysis suggests that the geochemistry of the ER water is mainly controlled by four factors: rock weathering or salt dissolution, dissolved CO2 partial pressure, water temperature (T) and evaporation. These factors explain more than 80 % of the total variance in the data matrix.












Similar content being viewed by others
References
Al-Heety EA, Turky AM, Al-Othman EM (2011) Physico-chemical assessment of Euphrates river between Heet and Ramadi cities. Iraq J Water Resour Protect 3:812–823. doi:10.4236/jwarp.2011.311091
Aly AIM, Froehlich K, Nada A, Awad M, Hamza M, Salem WM (1993) Study of environmental isotope distribution in the Aswan High Dam Lake (Egypt) for estimation of evaporation of lake water and its recharge to adjacent groundwater. J Environ Sci Health 15:37–49
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, Boca Raton, p 311
Clarke FW (1924) Data of geochemistry, Vth edition. USGS, Bull no 770
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702
Criss RE (1999) Principles of stable isotope distribution. Oxford University Press, New York, p 254
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
Davis JC (1986) Statistics and data analysis in geology. Wiley, New York, p 647
Frederickson GC, Criss RE (1999) Isotope hydrology and time constants of the unimpounded Meramec river basin, Missouri. Chem Geol 157:303–317
Gaillardet J, Dupre B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Garrels RM, Christ CL (1965) Solutions, minerals and equilibria. Harper and Row, New York
Garrels RM, Mackenzie FT (1971) Evolution of the sedimentary rocks. WW Norton, New York
Gat JR (1996) Oxygen and hydrogen isotopes in the hydrologic cycle. Annu Rev Earth Planet Sci 24:225–262
GERSAR-SCET (1977) Development of the lower Euphrates valley, Technical Report Zone 1. Ministry of Irrigation, Syrian Arab Republic, Damascus, p 266
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090
Gibson JJ, Edwards TWD (2002) Regional surface water balance and evaporation–transpiration partitioning from a stable isotope survey of lakes in northern Canada. Global Biogeochem Cycles. doi:10.1029/2001GB001839
Hem JD (1992) Study and interpretation of the chemical characteristics of natural waters. USGS, Water-Supply Paper, Reston 2254
Herrmann A, Fineke B, Schoniger M, Maloszewski P, Stichler W (1990) The environmental tracer approach as a tool for hydrological evaluation and regionalization of catchment systems. In: Proceedings on regionalization in hydrology, Ljubljana, IAHS Publ no 191, pp 45–58
IAEA (2005) Isotopes in the water cycle, Past, present and future of a developing science. In: Aggarwal PK, Gat JR, Froehlich KFO (eds) Vienna, Springer, The Netherlands, p 381
IAEA (2012) Monitoring isotopes in rivers: creation of the global network of isotopes in rivers (GNIR), results of a coordinated research project 2002–2006. IAEA-TECDOC-1673, Vienna, Austria, p 243
ICARDA (2006) The main soil and water conservation technologies in Syria, an overview. Internship at ICARDA, Nov/Dec. 2006, p 72
Işçen CF, Altin A, Senoğlu B, Yavuz HS (2009) Evaluation of surface water quality characteristics by using multivariate statistical techniques: a case study of the Euphrates river basin, Turkey. Environ Monit Assess 151(1–4):259–264. doi:10.1007/s10661-008-0267-9
Jansen JML, Painter RB (1974) Predicting sediment yield from climate and topography. J Hydrol 21:371–380
Kattan Z (1989) Géochimie et hydrologie et des eaux fluviales des bassins de la Moselle et de la Mossig, Transports dissous et particulaires, Cycles biogéochimiques des éléments. Thèse PhD, ULP Univ., Strasbourg, France, p 220
Kattan Z (2001) Use of hydrochemistry and environmental isotopes for evaluation of groundwater in the Paleogene limestone aquifer of the Ras Al-Ain area (Syrian Jezireh). Environ Geol 41:128–144
Kattan Z (2008) Estimation of evaporation and irrigation return flow in arid zones using stable isotope ratios and chloride mass-balance analysis: case of the Euphrates river, Syria. J Arid Environ 72(5):730–747
Kattan Z, Najjar H (2005) Groundwater salinity in the Khabour-Euphrates down-stream valleys. Groundwater and Saline Intrusion, Hidrogeología y Aguas Subterráneas, Instituto Geológico y Minero de España 15:565–583
Kempe S (1984) Sinks of the anthropogenically enhanced carbon cycle in surface fresh water. J Geophys Res 89:4657–4676
Kendall C, Coplen TB (2001) Distribution of oxygen-18 and deuterium in river waters across the United States. Hydrol Process 15:1363–1393
Livingstone DA (1963) Data of geochemistry (6th edn). USGS, Prof Paper 440-G
Martinelli LA, Gat JR, Camargo PB, Lara LL, Ometto JPHB (2004) The Piracicaba river basin: isotope hydrology of a tropical river basin under anthropogenic stress. Isotopes Environ Health Stud 40:45–56
Meybeck M (1979) Concentration des eaux fluviales en éléments majeurs et apports en solution aux océans. Revue Géologie Dynamique et Géographie Physique 21, fasc 3:215–246
Meybeck M (1983) Atmospheric inputs and river transport of dissolved substances. In: Proceedings of IAHS symposium on dissolved loads of river and surface water quantity and quality relationships, IAHS Publ no 141, Hamburg, pp 173–192
Meybeck M (1986) Composition chimique des ruisseaux non pollues en France. Sci Geol Bull, Strasbourg, France 39(1):3–77
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Milliman JD, Syvitski JPM (1992) Geomorphic/tectonic control of sediment discharge to the ocean, the importance of small mountainous rivers. J Geol 100:525–544
Odemis B, Evrendilek F (2007) Monitoring water quality and quantity of national watersheds in Turkey. Environ Monit Assess 133:215–229. doi:10.1007/s10661-006-9574-1
Ozturk F (1996) Suspended sediment yields of rivers in Turkey. In: Proceedings of the exeter symposium on erosion and sediment yield: global and regional perspectives, IAHS Publ no 236, pp 65–71
Payne BR, Quijano L, Latorre DC (1979) Environmental isotopes in a study of the origin of salinity of groundwater in the Mexicali Valley. J Hydrol 41:201–214
Plummer LN, Jones BF, Truesdell AH (1976) WATEQF- A FORTRAN IV version of WATEQ. Water Resour Invest USGS 13:61
Ponikarov VP (1967) The geology of Syria, explanatory notes on the map of Syria, Scale 1:500,000, part II, Mineral deposits and underground water resources. Technoexport, Moscow
Probst JL, Mortatti J, Tardy Y (1994) Carbon river fluxes and global weathering CO2 consumption in the Congo and Amazon River basins. Appl Geochem 3:1–13
Reeder SW, Hitchon B, Levinson AA (1972) Hydrogeochemistry of the surface waters of the Mackenzie River drainage basin, Canada-I. Factors controlling inorganic composition. Geochim Cosmochim Acta 36:825–865
Rosanski K, Araguas-Araguas L, Gonfiantini R (1993) Isotopic patterns in modern global precipitation. In: Swart PK, Lohmann KC, McKenzie J, Savin S (eds) Climate change in continental isotopic records. Am Geophys Union Monogr Series no 78, pp 1–36
Simpson HJ, Herczeg AL (1991) Stable isotope as an indicator of evaporation in the River Murray, Australia. Water Resour Res 27:1925–1935
Singh AK, Hasnain SI (2002) Aspects of weathering and solute acquisition processes controlling chemistry of sub-alpine proglacial streams of Garhwal Himalaya, India. Hydrol Process 16:835–849
Singh AK, Mondal GC, Singh PK, Singh S, Singh TB, Tewary BK (2005) Hydrochemistry of reservoirs of Damodar River basin, India: weathering processes and water quality assessment. Environ Geol 48:1014–1028. doi:10.1007/s00254-005-1302-6
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon 2. The influence of geology and weathering environment on the dissolved load. J Geophys Res C14(88):9671–9688
STATISTICA 6 (2007) STATISTICA 6 for windows 7 numerical analysis. StatSoft. http://www.statsoft.com
Stumm W, Morgan JJ (1981) Aquatic chemistry: an introduction emphasizing chemical equilibria in natural waters. Willey, New York, p 583
Swanson S, Bahr J, Schwar M, Potter K (2001) Two-way cluster analysis of geochemical data to constrain spring source waters. Chem Geol 179:73–91
Trondalen JM (2009) Climate changes, water security and possible remedies for the middle east, world water assessment programme side publications, March 2009, UNESCO-PCCP. http://www.unesco.org/water/wwap/pccp
Uhlenbrook S, Frey M, Leibundgut C, Maloszewski P (2002) Hydrograph separation in a mesoscale mountainous basin at event and seasonal timescales. Water Resour Res 38:1–14
UNEP (2001) The Mesopotamian Marshlands: demise of an ecosystem, division of early warning and assessment. United Nations Environment Programme (UNEP), Nairobi, Kenya. http://www.grid.unep.ch/activities/sustainable/tigris/marshlands
Vitvar T, Balderer W (1997) Estimation of mean residence times and runoff generation by 18O measurements in a Pre-Alpine catchment (Rietholzbach, eastern Switzerland). Appl Geochem 12:787–796
Winston WE, Criss RE (2003) Oxygen isotope and geochemical variations in the Missouri River. Environ Geol 43:546–556
Wollast R, Mackenzie FT (1983) The global cycle of silica. In: Aston SE (ed) Silicon geochemistry and biogeochemistry. Academic Press, London, pp 39–76
Zaidan T, Rahman I, Saaod W (2009) An environmental study of chemical and physical pollutants in Euphrates river water in Ramadi and Fallujah. J Univ Anbar Pure Sci 3(3):54–64
Acknowledgments
The author is grateful to Prof. I. Othman, Director General of AECS for his kind permission to publish this paper. Prof. W. Rasoul-Agha is deeply acknowledged for his comments and valuable remarks. The IAEA Organization, in particular, Mrs P.K. Aggarwal and T. Vitvar are gratefully acknowledged for financial assistance of the coordinated research project (contract-12643). Helps rendered by the technical staff of the Geology Department at AECS in analysis of water samples are also indebted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kattan, Z. Chemical and isotopic characteristics of the Euphrates River water, Syria: factors controlling its geochemistry. Environ Earth Sci 73, 4763–4778 (2015). https://doi.org/10.1007/s12665-014-3762-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3762-z