Abstract
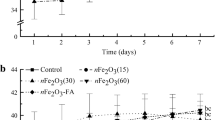
This study was conducted to determine the phytotoxicity of 6 nm γ-Fe2O3 nanoparticles (IONPs) in terms of root elongation and the physiological performance of rice plants. Rice seeds (Oryza sativa L. var. Koshihikari) exposed to IONPs at 500, 1,000 and 2,000 mg/L, had a significantly higher root elongation than the control and its bulk counterparts (IOBKs), indicating that the effect can be nanospecific. In a 14-week greenhouse pot experiment, the CO2 assimilation rate in IOBK and IONP-treated pots (500 and 1,000 mg/pot) decreased over time, with the decline (maximum 42.5 %) being less pronounced for IONPs, indicating that the effect cannot be inferred from the toxicity of nanoscale size iron oxide. Excessive adsorption of IONPs onto soil colloids with subsequent low water extractable iron was responsible for the unremarkable phytotoxic nature of IONPs in the rice plants. Amendment of IONPs coated with 20 mmol citric acid (IONPs-Cit) significantly diminished the CO2 assimilation rate and the decrease was similar to its bulk counterpart (IOBKs-Cit). However, maximum shoot growth inhibition (37 %) was associated with the application of IOBKs-Cit. It was concluded that massive accumulations of Fe plaque on the root surfaces of IOBKs-Cit treatments due to a decline in the pH of rhizoplane soils compared to the IONPs-Cit treatments were responsible for the remarkable shoot growth reduction. This study provided evidence of the phytotoxicity of γ-Fe2O3 nanoparticles, demonstrating the lower toxicity of nanosized iron oxide compared to a microsized preparation under reductive conditions.




Similar content being viewed by others
References
Alidoust D, Isoda A (2013), Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiol Plant. doi: 10.1007/s11738-013-1369-8
Alidoust D, Suzuki S, Matsumura S, Yoshida M (2012) Chemical speciation of heavy metals in the fractionated rhizosphere soils of sunflower cultivated in a humic Andosol. Commun Soil Sci Plant Anal 43(17):2314–2322
Arvizo RR, De M, Rotello VM (2007) Proteins and nanoparticles: covalent and noncovalent conjugates. In: Mirkin CA, Niemeyer CM (eds) Nanobiotechnology II: more concepts and applications. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Barrena E, Casals E, Colon J, Fon Xt, Sanchez A, Puntes V (2009) Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75:850–857
Becker M, Asch F (2005) Iron toxicity in rice-conditions and management concepts. J Plant Nutr Soil Sci 168:558–573
Bee A, Massart R, Neveu S (1995) Synthesis of very fine maghemite particles. J Magn Magn Mater 149:6–9
Bulte JW, Kraitchman DL (2004) Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 17(7):484–499
Colmer TD, Voesenek L (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Dehner CA, Barton L, Maurice PA, Dubois JL (2010) Size-dependent bioavailability of hematite (α-Fe2O3) nanoparticles to a common aerobic bacterium. Environ Sci Technol 45:977–983
Fauconnier N, Roger J, Pons JN (1996) Adsorption of gluconic and citric acids on maghemite particles in aqueous medium. Prog Colloid Polym Sci 100:212–216
Fox TR, Comerford NB, McFee WW (1990) Phosphorus and aluminium from a spodic horizon mediated by organic acids. Soil Sci Soc Am J 54:1763–1767
Ghodak G, Seo YD, Lee DS (2011) Hazardous phytotoxic nature of cobalt and zinc oxide nanoparticles assessed using Allium cepa. J Hazard Mater 186:952–955
Gobran GR, Clegg S (1996) A conceptual model for nutrient availability in the mineral soil-root system. Can J Soil Sci 76:125–131
He S, Feng Y, Ren H, Zhang Y, Gu N, Lin X (2011) The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J Soils Sed 1:1–10
Hinsinger P (2001) Trace elements in the rhizosphere. Bioavailability of trace elements as related to root-induced chemical changes in the rhizosphere, chapter 2. CRC Press LCC, Boca Raton, pp 25–41
Hoffmann WF, Barber SA (1971) Phosphorus uptake by wheat (Triticum aestivum) as influenced by ion accumulation in the rhizocylinder. Soil Sci 112:256–262
Jackson MB, Armstrong W (2009) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1:274–287
Jun YW, Lee JH, Cheon J (2007) Nanoparticle contrast agents for molecular magnetic resonance imaging. In: Mirkin CA, Niemeyer MC (eds) Nanobiotechnology II: more concepts and applications. Wiley-VCH, Weinheim
Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ASC Nano 3–10:3221–3227
Kotsmar C, Yoon KY, Yu H, Ryoo SY, Barth J, Shao S, Prodanovic M, Milner TE, Bryant SL, Huh C, Johnston KP (2010) Stable citrate-coated iron oxide superparamagnetic nanoclusters at high salinity. Ind Eng Chem Res 49:12435–12443
Kraemer SM (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquat Sci 66:3–18
Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108(6):2064–2110
Lei Z, Fashui H, Shipeng L, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–91
Marschner H, Treeby M, Römheld V (1989) Role of root-induced changes in the rhizosphere for iron acquisition in higher plants. Z Pflanzenernähr Bodenkd 152:197–204
Nowack B, Bucheli TD (2007) Occurence, behavior and effects of nanoparticles in the environment. Environ Pollut 150:5–22
Pereira EG, Olivaa MA, Rosado-Souzaa L, Mendesa GC, Colaresb DS, Stopatoa CH, Almeida AM (2013) Iron excess affects rice photosynthesis through stomatal and non-stomatal limitations. Plant Sci 201–202:81–92
Seeger EM, Baun A, Kästner M, Trapp S (2009) Insignificant acute toxicity of TiO2 nanoparticles to willow trees. J Soils Sediment 9:46–53
Sellers K, Mackay C, Bergeson LL, Clough SR, Hoyt M, Chen J, Henry K, Hamblen J (2009) Nanotechnology and the environment. CRC Press, Boca Raton
Trolard F, Tardy Y (1987) The stabilities of gibbsite, boehmite, aluminous goethite and aluminous hematites in bauxites, ferricretes and laterites as a function of water activity, temperature and particle size. Geochim Cosmochim Acta 51:945–957
US Environmental Protection Agency (1996) Ecological effects test guidelines (OPPTS 580.4200), seed germination/root elongation toxicity test
Wang M, Chen L, Chen S, Ma Y (2012) Alleviation of cadmium-induced root growth inhibition in crop seedlings by nanoparticles. Ecotoxicol Environ Safe 79:18–51
Zhang Y, Kallay N, Matijevic E (1985) Interactions of metal hydrous oxides with chelating agents. VII. Hematite-oxalic and citric acid systems. Langmuir 1:201–206
Acknowledgments
The authors wish to thank Masanori Hanata and Yosuke Miyauchi for their contribution to the laboratory works as well as for their technical supports. Special thanks go to Dr. Masayuki Kawahigashi from Tokyo Metropolitan University for his kind supports in determination of iron using ICP-AES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alidoust, D., Isoda, A. Phytotoxicity assessment of γ-Fe2O3 nanoparticles on root elongation and growth of rice plant. Environ Earth Sci 71, 5173–5182 (2014). https://doi.org/10.1007/s12665-013-2920-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2920-z