Abstract
To determine radioactivity and trace metal levels, surface sediments were collected from two important areas (İzmir Bay and Didim) in the Aegean Sea region of Turkey, and were analyzed for concentrations of 210Po, 210Pb and trace metals (Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn). The average 210Po and 210Pb massic activities in sediments varied in the range of 24 ± 5 to 126 ± 6 Bq kg−1 dry wt. and 18 ± 3 to 59 ± 4 Bq kg−1 dry wt., respectively. Izmir Bay exhibited the highest polonium activities in sediments, likely due to specific sedimentation processes and other sediment characteristics. The trace metal results showed that the Izmir Bay is facing trace metal pollution. The metal concentrations in sediment samples are low compared to those from the other neighboring marine environments.
Similar content being viewed by others
Introduction
The importance of monitoring radionuclides and trace metals is related to the impact of these elements on the marine environment. Several natural and artificial radionuclides have been used in environmental studies, especially in marine processes. 210Po and 210Pb are important natural radionuclides used in studies on the marine environment.
210Po (t 1/2 = 138 days), a high-energy α-particle emitter in the 238U decay chain, is a naturally occuring radionuclide formed by the beta decay of its grandparent of 210Pb (t 1/2 = 22.3 years) via 210Bi. The main source of 210Po and 210Pb is 222Rn emanation from the continents. In the aquatic environment, 210Po is largely produced from the decay of 210Pb deposited from the atmosphere (Stepnowski and Skwarzec 2000). The naturally occuring radionuclides 210Po and 210Pb are important because of their contributions to the natural radiation dose and technologically enhanced releases from sources of natural radioactivity (Vreček et al. 2004).
Coastal environments are subjected to metal contamination via inputs from main natural sources (rock weathering, soil erosion, dissolution of water-soluble salts), industrial and urban sources (municipal wastewater-treatment plants, manufacturing industries, and agricultural activities etc.) that are transported via river discharge and eolians processes (Güven and Akıncı 2008; Uluturhan et al. 2011).
Major indicators of pollution in aquatic environments are contaminated sediments. Sediments are the primary repository of radionuclides and chemicals entering the marine environment (Saçan et al. 2010). Thus, marine sediments are commonly used as environmental matrices in chemical and radioactive monitoring programs.
This study presents 210Po,210Pb and trace metal levels that were measured in marine sediments from two points in the Aegean Sea coast (Izmir Bay and Didim).
Materials and methods
Sample collection and preparation
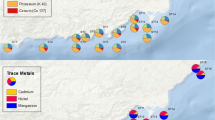
Surface marine sediment samples were collected using van-veen grab for trace metals and radionuclides during 2006–2007 from two stations in the coast of Aegean Sea every month. The locations of sampling stations are given in Fig. 1.
210Po and 210Pb measurements
The marine sediment samples were weighed and oven-dried to a constant weight at 80 °C and then thoroughly mixed. The samples were ground and passed through a 2 mm mesh followed by homogenization.
After the addition of a standardized amount of 209Po (4.88 MeV alpha emission, t 1/2 = 109 year) tracer, each sample was dissolved using three portions of concentrated 20 mL HNO3 and evaporated to near dryness on a hot plate at 55 °C. Then 2 mL H2O2 was added, and evaporated to near dryness. The sample residuals were treated with three portions of 20 mL HCl, and evaporated to near dryness. Finally, the samples were dissolved in 200 mL of 0.5 M HCl, and ascorbic acid of 4 mg was added to the plating solution to reduce iron. The temperature of the solution was kept constant at 70 °C while stirring, and a copper disk was introduced into the solution in such a way that only one side of the disk was available for plating. The polonium was spontaneously deposited onto the surface of the disk for 5 h at 70 °C. The copper disk was then removed, washed with distilled water and dried at room temperature (Flynn 1968).
210Po levels were measured via 5.30 MeV alpha particle emission rates using a high-resolution alpha spectrometer equipped with 450 mm2 Passivated Implanted Planar Silicon (PIPS) detector (Canberra Model 7401 Alpha PIPS dedector). The spectrometer was connected to a conventional personal computer (PC) using a network connection. The different parameter settings and the viewing of spectra were performed using the commercially available software (Genie-2000 Basic Spectroscopy Software). Contamination of detectors with polonium isotopes such as 210Po and 209Po probably occurs by some other process than alpha recoil. This is probably due to the inherent volatility of polonium at low pressure. Polonium activity is transferred from the sample sources to the detectors, a very serious problem with the long-lived Po-210 and even worse when working with Po-209 (t 1/2 = 102 y) as a yield tracer (Sill and Olsen 1970).
After the first deposition of 210Po, the residual 0.5 M HCl was kept for 1 year to allow 210Po in-growth from the 210Pb contained in the sample solution.
The samples were re-plated and the 210Po activities were determined. The second deposition provided information about the 210Pb content of the samples and thus indicated the extent to which the initial 210Po was supported by its grandparent species.
Well known Bateman equations were used to obtain 210Pb activity from measured 210Po activity.
Lower limit of detection (LLD) was calculated using the Currie definition (Currie, 1968). The concentration of 210Po in a small number of samples was below the detection limit, but most of the 210Po levels were within detection limits (0.0003 Bq). Counting period was adjusted to obtain relative standard error of approximately 5 %. Final activity calculations were attained to include the appropriate corrections for blanks and also for collection date. Recovery was obtained to vary between 70 and 81 % for samples.
Metal analysis
One gram of the sediment sample was dissolved in concentrated nitric acid in a Teflon beaker and small amount of hydrofluoric acid was added. 5 mL of concentrated H2SO4 was added on the sample and the beaker placed on the hot plate at 70–80 °C. After, a small amount of the concentrated HNO3 was added very slowly and continued heating at 120 °C. When the sample solution became liquid, hydrogen peroxide was added and heating continued at the same temperature for 30 min. The hydrogen peroxide was added until the sample became clear. After that, the sample was diluted to 100 mL with 2 % HNO3 in a volumetric flask (Topçuoğlu et al. 2002). Trace metal levels (Mn, Fe, Cr, Ni, Zn, Cu, Cd and Pb) were determined by a Perkin-Elmer inductively coupled plasma-optical emission spectrometry (ICP-OES).
Results and discussion
The activity concentrations of 210Po and 210Pb were determined within the range of 24 ± 5 to 126 ± 6 Bq kg−1 dry wt. with an average value of 67 ± 2 Bq kg−1 and 18 ± 3 to 59 ± 4 Bq kg−1 dry wt. with an average value of 37 ± 1 Bq kg−1, respectively. These values were comparable with those given in literature for sediments from different region in Aegean Sea as shown in Table 1. The activity of 210Po and 210Pb in marine sediment samples of Didim and Izmir Bay is also shown in Figs. 2 and 3. The activity of 210Po and 210Pb in sediment samples was found to be high in 210Po compared to 210Pb. The highest 210Po concentrations were measured in Didim (126 Bq kg−1) sediments. The possible source for enhanced 210Po, especially in Didim, is the discharge of Büyük Menderes river. The river flows into Didim station after carrying fertilized agricultural soil.
Likewise, Saçan et al. (2010) studied 210Po and 210Pb concentrations in sediment samples from the Izmir Bay (Aegean Sea), and determined that the highest 210Po activities were observed in winter and summer periods. They pointed out that an increase in 210Po concentrations has been observed in Izmir Bay, probably due to inputs from the Melez stream which is polluted by untreated wastewaters such as industrial, domestic and agricultural sources.
The 210Po/210Pb activity ratio is also investigated in the present study. The 210Po/210Pb ratio for Didim and Izmir Bay stations is found to be ranged from 0.83 to 4.03, and from 0.88 to 3.21, respectively. Also, the mean 210Po/210Pb ratio for Didim and Izmir Bay is determined to be 1.86 and 1.96, respectively. In general, the 210Po/210Pb activity ratios are higher than unity in the measured samples. In study, the highest 210Po/210Pb activity ratios were obtained in Didim for April.
The range of trace metal concentrations (μg g−1 dry wt.) in the Izmir Bay and Didim sediments (respectively) were: Cr 9–65 and 9–22, Cu 9–38 and 3–9, Fe 4709–18470 and 1271–11405, Mn 76–542 and 42–371, Ni 5–33 and 3–18, Pb 0–16 and BDL, Zn 17–85 and 3–30 μg g−1.
Minimum and maximum concentrations of trace metals (Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) determined in the sediments are presented in Table 2. The highest concentrations of metals were found in the Izmir Bay which is intensely industrialized (mainly iron, paper and pulp factories, antifouling paints, chlorine-alkali plants, chemical industries, textile industries, metal processing, timber processing, cement factories, tanneries, oil, soap and a very busy harbor) compared to Didim.
In the past, various studies were conducted to determine the trace metals in the sediments of Izmir Bay.
Metal concentrations (μg g−1 dry wt.) in sediments of Didim and Izmir Bay were compared to other studies in sediments from different regions of the world (Table 3). Comparison of data set revelated that observed trace metal levels in the Didim and Izmir Bay were generally lower than other regions. On the other hand, the levels of metals in this study were higher than Egypt. Different extraction procedures were used in the previous studies and this may have contributed to the differences (Güven and Akıncı 2008).
Conclusions
The following conclusions can be derived:
-
1.
The activity concentrations of 210Po and 210Pb were determined in the surface marine sediments in Izmir Bay and Didim (Aegean Sea).
-
2.
The activity concentrations of 210Po were determined within the range of 24 ± 5 to 126 ± 6 Bq kg−1 dry wt.
-
3.
The activity concentrations of 210Pb were determined within the range of 18 ± 3 to 59 ± 4 Bq kg−1 dry wt.
-
4.
The highest 210Po concentration was observed at the Didim station.
-
5.
210Pb activity concentrations in most of the sediment samples were lower than 210Po, and usually 210Po/210Pb activity ratios are much higher for natural levels.
-
6.
The overall order of the metal concentrations found in sediments in our study was: Fe > Mn > Zn > Cr > Cu > Ni > Pb > Cd. Our results show that the distribution of metals in the surface sediment is variable.
References
Accornero A, Gnerre R, Manfra L (2008) Sediment concentrations of trace metals in the Berre Lagoon (France): an assessment of contamination. Arch. Environ. Con. Tox. 54:372–385
Bellucci LG, Frignani M, Paolucci D, Ravanelli M (2002) Distribution of heavy metals in sediments of the Venice Lagoon: the role of the industrial area. Sci Total Environ 295:35–49
Boisson F, Miquel J-C, Cotret O, Fowler SW (2001) 210Po and 210Pb cycling in a hydrothermal vent zone in the coastal Aegean Sea. Sci Total Environ 281(1–3):111–119
Flynn WW (1968) The determination of low levels of polonium-210 in environmental materials. Anal Chim Acta 43:221–227
Küçüksegin F, Kontaş A, Uluturhan E (2011) Evaluations of heavy metal pollution in sediment and Mullus barbatus from the Izmir Bay (Eastern Aegean) during 1997–2009. Mar Pollut Bull 62:1562–1571
Güven DE, Akıncı G (2008) Heavy metals partitioning in the sediments of Izmir Inner Bay. J Environ Sci 20:413–418
Pekey H (2006) Heavy metal pollution assessment in sediments of the Izmit Bay. Turk Environ Monit Assess 123:219–231
Saçan S, Uğur A, Sunlu U, Büyükışık B, Aksu M, Sunlu FS (2010) The 210Po and 210Pb levels in surface sediment samples in the Izmir Bay (Aegean Sea-Turkey). Environ Monit Assess 161:575–582
Sarı E, Cağatay MN (2001) Distributions of heavy metals in the surface sediments of Gulf of Saros, NE Aegean Sea. Environ Inter 26:169–173
Sill CW, Olsen DG (1970) Sources and Prevention of recoil contamination of solid-state alpha detectors. Anal Chem 42:1596–1607
Stepnowski P, Skwarzec B (2000) Tissue and subcellular distributions of Po-210 in the crustacean Saduria entomon inhabiting the southern Baltic Sea. J Environ Radioactiv 49:195–199
Taher AG (2001) Geochemistry of recent marine sediments in the Bardawil lagoon, northern Sinai. Egypt. Hydrobiol. 457:5–16
Topçuoğlu S, Kırbaşoğlu Ç, Güngör N (2002) Heavy metals in organisms and sediments from Turkish Coast of the Black Sea, 1997–1998. Environ Int 27:521–526
Uğur (Tanbay) A, Yener G (2001) Accumulation rates and sediment deposition in the Gökova Bay in Aegean Sea Turkish Coast. Appl Radiat Isot 55:581–588
Uğur A, Miquel JC, Fowler SW, Appleby P (2003) Radiometric dating of sediment cores from a hydrothermal vent zone off Milos Island in the Aegean Sea. Sci Total Environ 307:203–214
Uluturhan E, Kontaş A, Can E (2011) Sediment concentrations of heavy metals in the Homa Lagoon (Eastern Aegean Sea): assessment of contamination and ecological risks. Mar Pollut Bull 62:1989–1997
Vreček P, Benedik L, Pihlar B (2004) Determination of 210Pb and 210Po in sediment and soil leachates and in biological materials using a Sr-resin column and evaluation of column. Appl Radiat Isot 60(5):717–723
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Aközcan, S., Uğur Görgün, A. Trace metal and radionuclide pollution in marine sediments of the Aegean Sea (Izmir Bay and Didim). Environ Earth Sci 69, 2351–2355 (2013). https://doi.org/10.1007/s12665-012-2064-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-2064-6