Abstract
Stored metallurgy and mining wastes contain relatively high amounts of potentially toxic elements. To monitor the distribution of contaminants originating from dumps, the chemical and physical properties of the wastes must be characterised. In this study, the chemical properties of wastes deposited in two different locations in Southern Poland (Szklary and Zloty Stok) were evaluated. Heaps located in Zloty Stok contain wastes from gold mineralisation comprising arsenic while wastes in Szklary originate from a factory that produced an iron-nickel alloy. In Szklary the total concentrations of Ca, Mg, Fe, Zn, Mn, Cr, Co, Cu, Ni, Tl, Ag, Cd and Pb were determined, while in Zloty Stok also As is an important contaminant. To assess the risk of contamination of the surrounding environment and to select the proper method for removing the contaminants, information on the distribution of elements between operationally defined phases must be obtained. For this purpose, a six-step sequential extraction was used. The mobility of most elements in the wastes from Szklary and Zloty Stok was relatively low; however, the large amount of As, 40–180 mg L−1, that could be released to environment in case of Zloty Stok was high. The results of fractionation studies indicated that Ag, Cd, Tl, Co, Cu, Cr, Pb, Zn and Mn may be released into environment under low pH and low redox potential conditions, which can be induced by bacterial activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heaps of industrial wastes containing various hazardous materials may be dangerous to the surrounding environment. Pollutants released from the heaps can contaminate the water, soil and air, and can affect human health. Therefore, areas where dangerous wastes are stored must be monitored to prevent contamination.
Industrial waste heaps can cause water pollution. For instance, contaminants such as metals, hydrocarbons, organic solvents and polycyclic aromatic carbons can be released from the heap. Salvarredy-Aranguren et al. (2008) investigated on the wastes from tin, zinc and lead mines in Milluni Valley, Bolivia, and discovered that both the surface and ground water of the surrounding area were highly contaminated with Cd, Zn, As and Pb. Woo and Choi (2001) investigated the water resources near a gold mine waste site in Korea and studied the distribution and behaviour of contaminants in the surface and groundwater. The results of the aforementioned study indicated that the concentration of As, Cd and Mn in the drinking water exceeded the guidelines of the WHO. Wastes from mining and smelting operations in Bukowno, Silesia region of Poland contaminated the water and sediments of the surrounding region (Krasnodebska-Ostrega et al. 2005a). Krysiak and Karczewska (2007) investigated the arsenic extractability of soils at an abandoned mining site in Zloty Stok and an arsenic mining area in Zelezniak (Southern Poland), and found that the arsenic concentration of the soil was high and that significant amounts of arsenic were mobile.
Lower Silesia is located in Southern Poland and was industrially developed in the 20th century. Numerous plants and mines were located in Lower Silesia, and waste material was often deposited in the vicinity of the factories. Szklary is a post-industrial region in Lower Silesia that contains several hazardous waste heaps. In particular, an iron-nickel alloy was produced in Szklary until the end of the 1970s. When alloy production ceased, mineral fertilizers were produced for several years, and the wastes were deposited on the same heap. Therefore, the heaps in Szklary contain high concentrations of elements such as iron, nickel, chromium and calcium. In the present investigation, the area surrounding an abandoned gold mine in Zloty Stok was also evaluated. Arsenic ores such as arsenopyrite and loellingite were mined in Zloty Stok until the 1960s. In addition, gold was recovered throughout the entire mining period, and arsenic was recovered from the 18th century until the 1960s for the production of arsenic (III) oxide. The results of previous studies indicated that the area surrounding the mine is highly contaminated with arsenic. Namely, wastes collected from the vicinity of the mine were found to be an important source of arsenic in the contamination of water and soils. For instance, water samples from the vicinity of the mine contained up to 4 mg L−1 of arsenic (Jedynak et al. 2008). However, in Poland, the concentration limit for As in drinking water is 0.01 mg L−1. In addition, two plant species collected in the region surrounding the mine contained up to 530 mg kg−1 of arsenic in their tissues (Jedynak et al. 2009).
Elements occur in the environment in various physico-chemical forms and it strongly influences their toxicity. Solid–liquid extraction is a powerful tool for the determination of elements’ binding capacities for soils, sediments and wastes. Several sequential extraction procedures can be used for that purpose (Krasnodebska-Ostrega and Kowalska 2003; Sahuquillo et al. 2003; Mossop and Davidson 2003; Krasnodebska-Ostrega et al. 2006). However, the literature about the applications of sequential extraction of metals in metallurgy waste samples is limited (Margui et al. 2004).
Thus, the aim of the present study was to characterise wastes deposited in two different locations in Southern Poland (Szklary and Zloty Stok). Namely, the total concentration of Ca, Mg, Fe, Zn, Mn, Cr, Co, Cu, Ni, Tl, Ag, Cd, Pb and As was determined. To evaluate the distribution of elements between operationally defined phases of the wastes, a six-step sequential extraction procedure was used. Post-industrial regions containing similar kind of wastes are located all over the world [e.g. in Spain (Moreno-Jiménez et al. 2010), Korea (Woo and Choi 2001), China (Wu et al. 2011), and Brazil (de Mello et al. 2006)]. Therefore, results obtained during described investigations are of worldwide interest and may be helpful in the assessment of environmental impact of similar kind of industrial areas.
Materials and methods
Sampling and sample preparation
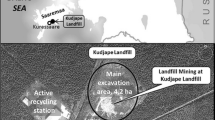
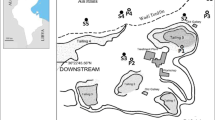
Waste samples were collected from two locations in the Lower Silesia region of Poland. The studied materials were heterogeneous due to differences in the production processes and the heterogeneity of the ores. Thus, samples were collected from many different parts of the waste dumps, and sampling points were selected according to the history of the manufacturers and the changes in the technological profile and the methods of ore processing. The first set of samples (samples 1–5) was collected from Szklary, which is located in the vicinity of Zabkowice Slaskie. Nickel ores occur naturally in the region and were processed in steelworks located in Szklary (N-50.645 E-16.825). The main product of ore processing is “half-finished” product, which was utilised for the production of Ni–Cr steel. Production was abandoned in 1979, and the plant produced Ca fertilizer during the final period of activity. Most of the heap contained metallurgical slag (sampling points 1, 2, 4 and 5), and a portion of the heap contained waste from the production of Ca fertilizer (sampling point 3). Samples 1 and 2 were collected from the southern region of the heap, samples 3 and 4 were collected from the western region of the heap and sample 5 was collected from the top of the heap. The second set of samples (samples 6–19) was collected from the neighbourhood surrounding an abandoned gold mine in Zloty Stok (N-50.439 E-16.875), which is located in Klodzko Valley, in the vicinity of the Golden Mountains. The site was rich in ores such as loellingite (FeAs2), arsenopyrite (FeAsS), pyrite (FeS2), chalcopyrite (CuFeS2), pyrrhotite (FeS) and galena (PbS). Moreover, the ores contained relatively large amounts of gold. The mine was closed in 1962. After production, wastes were deposited in several heaps; however, the majority of the wastes were deposited on the heap called Jan heap (N-50.437 E-16.854). Thus, samples 6–16 were collected from the Jan heap at distances of 10–100 m between sampling points. Sample 9 was collected from an area that was not affected by the plants. However, the waste at site 9 may have been green due to the presence of iron (III) arsenate (V), which is the product of loellingite oxidation. Sample 17 was collected from the heap next to black drift, while sample 18 was collected from an area containing large amounts of metallurgical slug. By contrast, sample 19 was collected from the heap next to “Gertruda drift”. The results of the preliminary study indicated that the samples were especially heterogeneous; thus, samples 1, 2, 3, 6, 9, 14 and 16 were conducted in duplicate (repeated samples were labelled as sample a or b).
A total amount of 1.5 kg of sample was collected from each point at a depth of 0–10 cm. Samples were air-dried, ground in an agate ball mill and stored in polypropylene containers at room temperature.
Reagents
A 0.43-mol L−1 solution of acetic acid was prepared by diluting 24.6 mL of glacial acetic acid (Merck) with water in a 1-L volumetric flask. To prepare a solution of 0.04-mol L−1 hydroxylamine hydrochloride in 25 % acetic acid, 7.5 g of NH2OH·HCl (puriss p.a.) (Fluka, UK) was dissolved in 250 mL of 100 % CH3COOH and was diluted with water in a 1-L volumetric flask. A 0.20-mol L−1 oxalate buffer was prepared by dissolving 24.8 g of NH4C2O4 and 18.1 g of H2C2O4 (both puriss p.a.) (Sigma–Aldrich, Germany) in a 1-L volumetric flask. To prepare an ammonium buffer (pH 9; 1 mol L−1), 10.0 g of NH4Cl (suprapur Merck) was dissolved in 20.0 mL of NH3 H2O (25 % p.a. POCh) and was diluted with water in a 200-mL volumetric flask. Standard solutions used in FAAS and ASV measurements were prepared by diluting Spectroscan solutions (1,000 mg L−1) of the appropriate element. Standard solutions used in ICP MS were prepared by diluting a multi element standard solution for ICP MS (10 mg L−1) (Merck, Germany). Ultrapure water obtained from a Milli-Q-Water System (Millipore, USA) was used throughout the study. Nitric acid, acetic acid, perchloric acid and hydrofluoric acid were suprapur grade and were purchased from Merck (Germany). In addition, 30 % hydrogen peroxide (Sigma–Aldrich, Germany) and dimethylglyoxime (DMG) (POCh) were used.
Instrumentation
The concentrations of Ca, Fe, Mg, Mn and Zn in samples and extracts were determined by a flame atomic absorption spectrometry (3110 Perkin Elmer, USA), and the concentrations of Cr, Co, Cu, Ni, Tl, Ag, Cd, Pb and As were measured with an Elan 6100 DRC inductively coupled plasma mass spectrometer (Perkin Elmer SCIEX, Canada). In addition, Cd, Cu, Pb and Zn were measured by anodic stripping voltammetry (ASV) with an μAUTOLAB electrochemical analyser (Autolab, Holland) equipped with a hanging mercury drop electrode (HMDE) as the working electrode, a saturated Ag/AgCl reference electrode and an auxiliary platinum electrode. To digest the samples, a Microwave Digestion System Ethos 1 (Milestone, Italy) was used. An Elpan 357 water bath shaker (Elpan, Poland) was used during the extractions. The total concentration of Al, C, Ca, Cr, Fe, K, Mg, Na, Ni, O, S, Si and Ti was determined with a scanning electron microscope equipped with an energy dispersive spectroscopy analyser (Zeiss, LEO 435 VP, Röntec M1, Germany). Samples before analysis were mixed with stearic acid dissolved in hexane. In the next step the pellet was formed. In SEM–EDX determination, carbon was used as a coating material.
Digestion of samples
Approximately 200 mg of homogeneous, dried material and a mixture of concentrated acids (2 mL of HNO3 and 1 mL HClO4) were placed in PTFE vessels and digested in a microwave digestion system. A three-stage program with a maximum temperature of 200 °C and maximum microwave power of 1,000 W was applied. In the second step, 0.5 mL of HF was added, and the three-stage program was re-applied. Digested samples were transferred into 50-mL volumetric flasks and diluted with Milli-Q water. All of the samples were processed in triplicate.
Extraction procedure
The chemical characterisation of the waste was based on a six-step sequential extraction procedure. In each step of the extraction procedure, 1 g of dried solid waste was extracted with 50 mL of the extractant in a 120-mL polyethylene container (steps 1–5). The extracts were centrifuged at 2,000 rpm for 30 min and were filtered through a 0.45-μm cellulose acetate membrane into a polyethylene container. After filtration, the extracts were acidified with 50 μL of concentrated HNO3 (to a pH 2) and were stored at 4 °C. The extractions were conducted in triplicate. The overall extraction scheme is presented in Table 1.
Quality of analytical results
To assess the quality of the results obtained by ICP MS, the concentrations of Co, Cu, Ni, Pb and Zn were determined by voltammetry. Cu, Pb and Zn were determined by ASV at a hanging mercury drop electrode under conditions described before (Krasnodebska-Ostrega et al. 2005b) and Co and Ni were determined by adsorptive stripping voltammetry (AdSV) according published procedure (Farghaly and Ghandour 2005). To estimate differences between the results obtained by spectrometric and voltammetric techniques, a t test (two-tailed P = 95 %, n 1 − 1 = 2; n 2 − 1 = 2; F crit = 39.00; n 1 + n 2 − 2 = 4; t crit = 2.78) was conducted on the mean values. Both sets of results did not differ significantly (t exp < t crit). The t exp value was in the range from 0.57 to 1.98. The results obtained from spectrometric and voltammetric tests were not significantly different.
The applicability of the analytical procedures used for the sample digestion as well as the applicability of the ICP MS method used for determination of selected elements were confirmed by analysing the certified reference materials (Fly Ash CTA-FFA-1 and Montana Soil 2710). The averaged results obtained from ICP MS were compared with known certified values (P = 95 %, n − 1 = 2, t crit = 4.30) applying a two-tailed significance test and no significant difference was found (t exp value was in the range from 0.173 to 4.23).
Results and discussion
Total element determination
Metallurgical wastes from Szklary
In the first step of the investigation, the concentration of macroelements was determined by SEM EDS, and the results are presented in Table 2. In samples collected from different sampling points, the concentration of the elements varied significantly. Thus, the results indicated that the wastes were not homogeneous. The heterogeneity of the wastes was attributed to the fact that wastes were deposited during various time periods, when ores were processed differently. Samples collected from Szklary contained 6.6–25 % C, 3.9–7.0 % Fe and relatively high concentrations of Ni and Cr (up to 0.6 and 0.3 %, respectively), which were associated with the production of Ni–Cr steel.
The determination of Ca, Mg, Mn and Fe was achieved with FAAS, and the results are presented in Table 3. All of the samples contained relatively high amounts of Ca, Mg and Fe. Sample 3 was collected from the site of Ca fertilizer waste deposition. As a result, sample 3 contained the highest concentration of Ca (173 g kg−1). By contrast, the concentration of Mn in waste from Szklary was less than 2 g kg−1. The Mn and Fe concentrations of samples collected from the same sampling points (Table 3) were compared, and the results indicated that the concentration of Mn and Fe was not significantly different. Moreover, significant differences in the Mg and Ca concentrations were not observed.
ICP MS was used to determine the concentration of trace elements (Ag, Cr, Co, Cu, Cd, Ni, Pb, Tl and Zn) in the samples (Table 4). The results indicated that the concentration of trace elements between samples was highly variable. For instance, sample 3 contained the lowest concentration of Pb and Ni. However, the chromium content obtained by ICP MS was not significantly different from the results obtained with SEM EDS. Moreover, the results obtained from samples collected from the same sampling points (Table 4) were compared, and significant differences among samples were not detected, with the exception of sample 1. Samples collected in Szklary contained relatively high concentrations of thallium (up to 1.1 mg kg−1 in sample 1), which is extremely toxic Table 4.
Mining wastes from Zloty Stok
In samples collected from Zloty Stok, the total concentration of macroelements was determined by SEM EDS, and the results are presented in Table 5. Among samples, the concentration of As, Fe and Cr was highly variable. For instance, the highest As concentrations were observed in samples 9 (approx. 10 %), 6 (approx. 5 %) and 16 (approx. 2.5 %). However, the content of mineral matrix elements (O, Al, C, S and Si) in all investigated samples was similar. The Ca, Mg, Mn and Fe content, which was determined by FAAS, is presented in Table 6. The results indicated that the concentration of Ca, Mg, Mn and Fe was strongly dependent on the sampling point. For instance, the concentration of Ca, Mg and Fe was relatively low in samples 10 and 11, which were collected from the Jan heap. The concentration of As, Ag, Cr, Co, Cu, Cd, Ni, Pb, Tl and Zn, which was determined by ICP MS, is presented in Table 7. In the majority of the samples collected from Zloty Stok, the concentration of Cr, Tl and Cd was similar, with the exception of samples 9, 11, 15, 18 and 19. Sample 18, which was collected from the site of metallurgical treatment deposition, contained the lowest amount of As. By contrast, the concentration of Ni and Cu in sample 6 was higher than that of the other samples. A comparison of the results obtained during determination of Pb, Ag, As, As and Cu in samples collected from the same sampling point revealed that the wastes were heterogeneous. The concentration of Au in the wastes from studied region, the gold mining area in Zloty Stok, is less than 0.5 mg kg−1 (Łuszczkiewicz 2006). Total concentration of arsenic obtained for wastes collected in Zloty Stok were compared with the results obtained for mine wastes and soils from Zloty Stok and Zelezniak. Total concentration of arsenic in the waste and soil samples investigated by Krysiak and Karczewska (2007) amounted to 270–43,500 mg kg−1. Concentration of arsenic in samples from Jan Heap amounted to 500–92,500 mg kg−1.
Fractionation study of solid metallurgy and mining wastes
To evaluate the chemical properties of different mineral phases and the distribution of selected elements between phases, a fractionation study was performed. Applied sequential extraction procedure was worked out to be optimal for the extraction of investigated samples. Several significant modifications must have been introduced to the original Tessier’s procedure to fulfil the intended purpose, for example H2O was applied in the 1st step of sequential extraction procedure instead of MgCl2 to compare the results with EP test which allows to classify the wastes as hazardous materials; acetic acid was used in the 2nd step of sequential extraction procedure instead of sodium acetate because it is the most efficient reagent to leach carbonate fraction. In addition, the reducible fraction was split into two fractions: easily reducible (hydroxylamine hydrochloride in 25 % acetic acid) and moderately reducible (oxalate buffer). The optimisation of the extraction procedure was described in details in our previous article (Krasnodebska-Ostrega et al. 2009). The six-step extraction procedure is presented in Table 1. Water was used in the first step of the extraction process to determine highly mobile fraction. The first step was chosen according to the results of popular leaching tests (e.g. extraction procedure test, EP). In the second step of the extraction procedure, a 0.43-mol L−1 solution of acetic acid, which can dissolve carbonate minerals, was used to determine carbonate fraction. Alternatively, in the third step of the extraction process, a 0.04-mol L−1 solution of NH2OH·HCl in 25 % HAc, which leaches elements bound to MnOx, was used to determine easily reducible fraction. A 0.20-mol L−1 solution of oxalate buffer, which leaches elements bound to FeOx, was used in the 4th step of the process to determine moderately reducible fraction. To leach the organic and sulphide fraction, a solution of 30 % H2O2 acidified to pH 2 with nitric acid was used as an extractant in the 5th step of the extraction process. Complete digestion was achieved with a mixture of HClO4, HNO3 and HF to estimate the concentration of elements bound to residue. The sequential extraction procedure was performed in triplicate, and the results are provided as the mean concentration of elements per kg of dry mass, along with the standard deviation (Tab. 8 supplementary material). The distribution of elements (their extractability) is presented as a percentage of the total concentration. The extractability was calculated as the ratio of the concentration of the element in each extract to the total concentration of the element after sample digestion. Fig. 1.
Fractionation study on samples collected from Szklary
The fractionation study revealed that the distribution of the elements followed two different trends. Based on the fractionation results, two samples (1 and 3) with different distribution of elements between operationally defined phases were selected, and the results for these samples are discussed in detail. The distribution of elements between operationally defined phases in sample 1 is presented in Fig. 2. The sampling point was located in the southern part of the heap, where metallurgical slag was deposited. In sample 1, 45 % Ca, 40 % Mn and 25 % Fe were extracted with acetic acid. The amount of Mn extracted with acetic acid was large due to the presence of Mg/Ca carbonates. In particular, Mn2+ can substitute for Mg2+ and Ca2+ in carbonate minerals such as dolomite and calcite (Arunachalm et al. 1996), respectively. More than 85 % of the total Mg content was leached with the mineral acids (the 6th step of sequential extraction). Thus, Mg was likely associated with the silicate and aluminosilicate matrix. In specific, SEM measurements confirmed that the Si and Al contents of the sample matrix were high. Moreover, although 15 % of the total Fe concentration was leached with the oxalate buffer solution, the majority of Fe (60 %) was found in the residual fraction. These results indicated that a minority of the total Fe content was associated with the reducible phase (Fe oxide). Sample 3 was collected from the western part of the heap, where wastes from the production of Ca fertilizers (CaCO3) were deposited. Therefore, high amounts of Ca (~90 %) were leached with acetic acid in the 2nd step of the extraction process (carbonate fraction). The distribution of elements between operationally defined phases is presented in Fig. 3. The highest amount of Mg (~90 %) was extracted in the final step (mineral acids leaching) of the extraction process, and the highest amount of Mn (57 %) was extracted with acetic acid. These results indicated that the majority of Mn was associated with carbonate minerals and reducible fractions. By contrast, more than 50 % of the total Fe content was leached in the 4th step of the process, and 12 % of the total Fe amount was extracted with a hot mixture of mineral acids. In sample 1, the highest amounts of Cd (63 %), Tl (50 %) and Pb (40 %) were extracted with acetic acid, while Ag (46 %) was mainly extracted with hydroxylamine hydrochloride in HAc (Fig. 2). Significant amounts of Ag (45 %), Cd (28 %), Pb (52 %) and Tl (21 %) were obtained in the final step of the extraction procedure. The distribution of elements between operationally defined phases suggested that large amounts of Ag, Cd, Tl and Pb were bound to acetic acid with and without hydroxylamine hydrochloride leachable fractions (2nd and 3rd step). Although the residue contained significant amounts of the elements (the highest amounts of Co, Cu, Cr and Ni were leached in the final step of the extraction process), Co, Cu, Cr and Ni in the wastes were not mobile. The highest concentration of Zn was obtained in the 2nd step of the extraction procedure due to the substitution of Mg2+ and Ca2+ in carbonate minerals. Relatively high concentrations of Co, Cu, Cr, Ni and Zn were found in fractions leached with reducible reagents and acetic acid, indicating that approximately 40–50 % of the total content of the elements was bound to carbonate and reducible phases (Fig. 2).
In sample 3, the highest amount of Pb (78 %) was leached with hydroxylamine hydrochloride, while high amounts of Cd were extracted in the 2nd (40 %) and 3rd (48 %) step of the extraction process (Fig. 3). These results indicated that Pb and Cd may be associated with carbonate and Mn-oxide minerals. In contrast to sample 1, considerable amounts of Ag, Tl and Cd were leached with 30 % H2O2, indicating that these elements were bound to an oxidisable phase. The extremely low extractability of metals in the 2nd step of the process and the high extractability of metals in the 5th step are indirect evidence of the presence of sulphide minerals or/and organic matter. The highest content of Zn was obtained in the 2nd (55 %) and 3rd (44 %) step of the extraction process, and significant amounts of leachable Cu were collected with reducible reagents (54 %). By contrast, considerable amounts of Cr were leached in the 2nd (40 %) and 4th (25 %) step. Moreover, significant amounts of Ni were leached with a mixture of concentrated mineral acids. Thus, the results suggested that Co, Cu, Cr, and Zn were bound to carbonate and reducible phases.
Based on the mineralogical composition of studied wastes (Wróbel et al. 2009) and distribution of the elements between operationally defined phases in wastes collected from Szklary, we can conclude that Ca was leached from calcite (sample 3) and oldhamit, Mg from pyroxene, Mn from calcite and pirolusite, and Fe from goethite, haematite and magnetite. Cd, Pb, Tl Co, Cu, Cr and Zn have a strong affinity to Fe- and Mn-oxides and carbonate minerals. The highest amounts of Co, Cu, Cr and Ni (immobile fraction) are bounded to silica minerals.
Fractionation study on samples collected from Zloty Stok
A fractionation study was conducted on all of the collected samples, and the results revealed that the distribution of the elements followed three different trends. Based on the results, three samples (6, 9 and 18) with different distributions between operationally defined phases were selected, and the results of these samples are discussed in detail. Sampling point 6 was located in the Jan heap, and the highest amounts of Ca were eluted with acetic acid (70 %) and a solution of hydroxylamine hydrochloride (30 %) (Fig. 4). Crystalline forms of carbonate minerals may not fully be dissolved in the 2nd step of the extraction process, using 0.43-mol/L acetic acid. However, remaining calcites are dissolved in the 3rd step due to the presence of high concentrations of acetic acid (25 %). In sample 6, 90 % of the total Mg content was leached with mineral acids. SEM measurements confirmed that relatively high amounts of Si and Al were present in the sample matrix; thus, Mg was likely associated with the silicate and aluminosilicate phase. The highest content of Fe was observed in moderately reducible (27 %) and residual (59 %) fractions (4th and 6th step), and oxalate buffer can completely dissolve amorphous and crystalline Fe oxides and hydroxides (Gleyzes et al. 2002). By contrast, the largest amounts of Mn were leached in the carbonate (30 %), easily reducible (20 %) and reducible (36 %) fraction, which is typical for this element. Similar tendencies were observed in other samples obtained from Zloty Stok. However, the distribution of elements in sample 9 was different from that of the other samples. Sampling point 9 was located in the Jan heap; however, the nature of the waste in that point was different from that of the other samples (the sample was green). In sample 9, the highest amount of Ca (73 %) (Fig. 5) was extracted with a mixture of mineral acids. The results of fractionation and SEM studies indicated that Ca in sample 9 was bound to silicates and aluminosilicates. Moreover, the distribution of Ca, Mg, Fe and Mn (Fig. 6) in sample 18 was similar to that of sample 9. However, sample 18 was not collected from the site of metallurgical slug deposition. The fractionation results of Co, Cu, Cr, Ni and Zn in samples 6 (Fig. 4), 9 (Fig. 5) and 18 (Fig. 6) indicated that the highest amount of Ni (81, 80 and 76 %, respectively) was leached with mixture of minerals acids in the last step. Thus, Ni may be permanently associated with the sample matrix and may not be released into the environment. Significant amounts of Co, Cu, Cr and Zn (20–30 %) were leached in the 2nd, 3rd and 4th step of the extraction procedure, indicating that these elements were bound to carbonate and reducible phases. These elements may be easily mobilised under low pH and low redox potential conditions. Their mobility can be also influenced by bacterial activity. In sample 6 (Fig. 4), the highest amounts of As (87 %) and Ag (79 %) were leached in the last step (mineral acids) of the applied extraction procedure. Arsenic minerals such as arsenopyrite and loellingite can only be dissolved with mineral acids. A similar distribution of As (74 %) and Ag (86 %) was observed in sample 9 (Fig. 5). Nevertheless, in sample 18, only 35 % of As (Fig. 6) was leached from the residual phase due to differences in the ore treatment process. The results obtained by Krysiak and Karczewska (2007) confirmed that arsenic in wastes collected from Zloty Stok is not mobile. However, authors applied different sequential extraction procedures. It was stated that arsenic contained in wastes is practically not mobile and arsenic in soils is mainly bounded to amorphous Fe oxides. The conclusion that approximately 90 % of As in investigated wastes is not mobile is consistent with our results. The mobile part of arsenic is bound to carbonates and amorphous Fe oxides. Arsenic preliminary leached from loellingite and arsenopyrite is probably adsorbed on carbonates and amorphous Fe oxides. Therefore, in the first step of sequential extraction procedure, in fraction leached with water, the concentration of arsenic was greater than 35 mg L−1. According to the EP test (test based on the extraction procedure using water), the highest permissible As concentration is 5 mg L−1. Therefore, wastes from Zloty Stok are considered dangerous (Baba and Kaya 2004). Arsenic leached from arsenopyrite, which is present at Zloty Stok in high concentrations, may be associated with carbonate and Fe-oxide minerals. Comparable results were obtained by Woo and Choi (2001), who investigated a similar area (a gold mine in Korea).
In total, 70 % of the Pb in sample 6 was leached with a mixture of concentrated mineral acids, 8 % was extracted with a solution of hydroxylamine hydrochloride and 15 % was extracted with oxalate buffer. In samples 9 (Fig. 5) and 18 (Fig. 6), 70 % and 50 % of the total Pb content was leached in the 3rd step of the extraction procedure, respectively. Alternatively, only 20 % of Pb was leached with mineral acids. The Pb fractionation results indicated that lead may be associated with a reducible phase. Significant amounts of Cd and Co were leached from both samples with acetic acid (2nd step), a solution of hydroxylamine hydrochloride (3rd step) and oxalate buffer (4th step), indicating that these elements were associated with carbonate and reducible phases. Tl was leached from sample 6 (Fig. 4) in the 3rd (over 50 %), 4th (over 20 %) and 6th (25 %) step of the extraction procedure. Thus, relatively high amount of Tl may be released into the environment under low pH and low redox potential conditions, which may be associated with bacterial activity. Similar trends in the Tl distribution were observed in samples 9 (Fig. 5) and 18 (Fig. 6). However, significant amounts of Tl, Cu, Cr, Zn, Co and Cd were leached from samples 9 and 18 with 30 % hydrogen peroxide, indicating that these elements may be bound to primary sulphides or organic matter.
Based on the information about the mineralogical composition studied wastes (Łuszczkiewicz and Muszer 1997; Grobelski and Farbiszewska-Kiczma 2008; Wróbel et al. 2009) and distribution of the elements between operationally defined phases in wastes collected from Zloty Stok, we can conclude that Ca was leached from calcite (sample 6) and from actinolite (samples 9 and 18), Mg from chrysotile and actinolite, Mn from calcite and pyrolusite, and Fe from goethite, loellingite, chalcopyrite and pyrite; As occurs in the form of loellingite and arsenopyrite. However, arsenic initially leached from loellingite and arsenopyrite is then associated with carbonates and amorphous Fe oxides. Cd, Pb, Tl, Co, Cu, Cr and Zn have a strong affinity to Fe- and Mn-oxides and carbonate minerals. Significant amounts of Tl, Cr, Zn, Co and Cd are bounded to sulphide minerals, Cu occurs in wastes in the form of chalcopyrite.
Conclusions
The aim of the present study was to characterise wastes deposited at two different locations in Southern Poland (Szklary and Zloty Stok). Wastes collected from heaps in Szklary and Zloty Stok were heterogeneous due to differences in the production processes and the heterogeneity of the ores. In the past, wastes were mainly characterised based on the determination of total content of harmful elements. Nowadays, the information about potential risks of environmental contamination should be connected with mobility of the elements present in the wastes. The distribution of elements between operationally defined phases gives information about environmental conditions under which the elements could be mobilised. The sequential extraction procedure is used to evaluate cost-effectiveness of recovery of valuable elements, to propose a suitable remediation method, even, to select the type of bacteria applied in biometalurgical processes; it is known that autotrophic bacteria leach elements bounded to sulphide minerals (Bosecker 1997).
In spite of differences (mineralogical composition, origin, different treatments) between wastes from Szklary and Zloty Stok, some similarities in geochemical behaviour of several elements were observed. In both investigated wastes, Mg was mainly associated with aluminosilicate and silicate minerals. Therefore, in these particular environments Mg is not mobile. In contrast, Ca is rather mobile in the wastes collected from both areas and the element occurs in the form of calcite, while only the small part was bound with silicate minerals. Fe occurs in different forms in both investigated types of wastes. Wastes collected from Szklary contain: goethite, hematite and magnetite and from Zloty Stok: goethite, loellingite, chalcopyrite and pyrite. Moreover, it should be pointed that long-term processes taking place in the environment, like pedogenesis, changes of pH or redox potential, caused partial transformation of primary Fe minerals into secondary minerals, amorphous forms of Fe hydroxides and Fe oxides. Mn in both investigated wastes occurs in similar forms: calcite and pyrolusite. These preliminary minerals could be transformed into secondary minerals in pedogenesis. Silver in general, independent of the wastes, occurs in the form of not mobile compounds. The only mobile part of that element was associated with secondary Fe and Mn oxides. In the investigated wastes, Zn and Cd were associated with Mn- and Fe-oxides and carbonate minerals in some extent (30–90 %) could be mobilised in the environment. The majority of Ni (70–90 %) is practically not mobile. Minor part of that element could be bound with or adsorbed on carbonates and Fe-oxide secondary minerals. Despite the differences between the primary geochemical forms of Cr in both types of wastes, the mobile forms of Cr (30–75 %) were mainly associated with carbonates and Fe–Mn secondary minerals. Similar chemical behaviour was observed in case of Co, therefore their similar geochemistry could be presumed. The mobile fraction of Cu and Tl is mainly associated with carbonates and secondary Fe–Mn minerals. While Pb in metallurgical wastes from Szklary is associated only with carbonates and in wastes after Ca fertilizer production is associated with carbonates and secondary Fe–Mn minerals. Only the minority of leachable fraction of Cu, Tl and Pb (chalcophile elements) could be released from sulphide minerals.
Arsenic in wastes from Zloty Stok is generally not mobile. Only a small part of total content of As, 2 %, could be leached from carbonates and amorphous Fe oxides. Nevertheless, it should be pointed out that total content of that element in pore water is really very high (500–92,500 mg/kg) and only 2 % of mobile fraction gives up to 35 mg L−1 of that element.
The distribution (chemical characterisation) of the elements between operationally defined phases was dependent on the sampling point. The mobility of the elements (the first step of sequential extraction with water) in wastes collected from Szklary and Zloty Stok was relatively low. However, the mobility of arsenic was so high that the concentration of that element exceeded the permissible concentration evaluated by the EP test (Baba and Kaya 2004); therefore, wastes from Zloty Stok are considered dangerous.
References
Arunachalm J, Emons H, Krasnodebska B, Mohl (1996) Sequential extraction studies on homogenized forest soil samples. Sci Total Environ 181:147–159
Baba A, Kaya A (2004) Leaching characteristic of solid wastes from thermal plants of western Turkey and comparison of toxicity methodologies. J Environ Manage 73:199–207
Bosecker K (1997) Bioleaching: metal solubilization by microorganisms. FEMS Microbiol Rev 20:591–604
de Mello JWV, Roy WR, Talbott JL, Stucki JW (2006) Mineralogy and arsenic mobility in arsenic-rich Brazilian soils and sediments. J Soils Sediments 6(1):9–19
Farghaly OA, Ghandour MA (2005) Square-wave stripping voltammetry for determination of eighth heavy metals in soil and indoor-airborne particulate matter. Environ Res 97:229–235
Gleyzes Ch, Tellier S, Astruc M (2002) Fractionation studies of elements in contaminated soils and sediments: a review of sequential extraction procedures. Trends Anal Chem 21:451–467
Grobelski T, Farbiszewska-Kiczma J (2008) Detoxification of the wastes heaps of former arsenopyrite ore mine. Chemia Dydaktyka Ekologia Metrologia 13(1–2):111–113
Jedynak L, Kieronski P, Kowalska J, Golimowski J (2008) Speciation analysis of arsenic by HPLC–UV in highly contaminated water samples. Chem Anal (Warsaw) 53:557–568
Jedynak L, Kowalska J, Harasimowicz J, Golimowski J (2009) Speciation analysis of arsenic in terrestrial plants from arsenic contaminated area. Sci Total Environ 407:945–952
Krasnodebska-Ostrega B, Kowalska J (2003) Ultrasound-assisted acetic acid extraction of metals from soils. Chem Anal (Warsaw) 48:967–974
Krasnodebska-Ostrega B, Dmowski K, Stryjewska E, Golimowski J (2005a) Determination of thallium and other elements (As, Cd, Cu, Mn, Pb, Se, Sb, and Zn) in water and sediment samples from the vicinity of the zinc-lead smelter in Poland. J Soil Sediment 5:71–73
Krasnodebska-Ostrega B, Stryjewska E, Golimowski J (2005b) Voltammetric determination of thallium and lead in sediment samples. Chem Anal (Warsaw) 50:807–810
Krasnodebska-Ostrega B, Kaczorowska M, Golimowski J (2006) Ultrasound-assisted extraction for the evaluation of element mobility in bottom sediment collected at mining and smelting Pb–Zn ores area in Poland. Microchim Acta 154:39–43
Krasnodebska-Ostrega B, Paldyna J, Kowalska J, Jedynak L, Golimowski J (2009) Fractionation study in bioleached metallurgy wastes using six-step sequential extraction. J Hazard Mater 167:128–135
Krysiak A, Karczewska A (2007) Arsenic extractability in the areas of former arsenic mining and smelting, SW Poland. Sci Total Environ 379:190–200
Łuszczkiewicz A (2006) Badania odpadów technologicznych z dawnej działalności górniczej i hutniczej w rejonie Złotego Stoku. (The study on technological wastes from mining and metallurgical industry from Zloty Stok area) Prace Naukowe Instytutu Górnictwa Politechniki Wrocławskiej 117:179–191
Łuszczkiewicz A, Muszer A (1997) Gold in mine wastes from Złoty Stok region (SW Poland). Physicochem Probl Miner Process 31:197–209
Margui E, Salvadó V, Queralt I, Hidalgo M (2004) Comparison of three stage sequential extraction and toxicity characteristic leaching tests to evaluate metal mobility in mining wastes. Anal Chim Acta 524:151–159
Moreno-Jiménez E, Manzano R, Esteban E, Peñalosa J (2010) The fate of arsenic in soils adjacent to an old mine site (Bustarviejo, Spain): mobility and transfer to native flora. J Soils Sediments 10:301–312
Mossop KF, Davidson CM (2003) Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal Chim Acta 478:111–118
Sahuquillo A, Rigol A, Rauret G (2003) Overview of the use of leaching/extraction tests for risk assessment of trace metals in contaminated soils and sediments. Trends Anal Chem 22:152–159
Salvarredy-Aranguren MM, Probst A, Roulet M, Isaure M (2008) Contamination of surface waters by mining wastes in the Milluni Valley (Cordillera Real, Bolivia): mineralogical and hydrological influences. Appl Geochem 23:1299–1324
Woo NC, Choi MJ (2001) Arsenic and metal contamination of water resources from mining wastes in Korea. Environ Geol 40:305–311
Wróbel I, Polowczyk I, Sadowski Z (2009) Transport cząstek koloidalnych z zaadsorbowanymi jonami arsenu przez mineralne złoże porowate (Transport of colloidal particles with arsenic ions adsorbed on the colloidal surfaces through mineral porous bed). Środkowo-Pomorskie Towarzystwo Naukowe Ochrony Środowiska 11:1120–1130
Wu Y, Xu Y, Zhang J, Hu S, Liu K (2011) Heavy metals pollution and the identification of their sources in soil over Xiaoqinling gold-mining region, Shaanxi, China. Environ Earth Sci 64:1585–1592
Acknowledgments
This study was supported by Grant K 118/T09/2005.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Paldyna, J., Krasnodebska-Ostrega, B., Kregielewska, K. et al. The assessment of environmental pollution caused by mining and metallurgy wastes from highly polluted post-industrial regions in Southern Poland. Environ Earth Sci 68, 439–450 (2013). https://doi.org/10.1007/s12665-012-1750-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1750-8