Abstract
Background
The decision to withdraw anti-tumor necrosis factor (anti-TNF) therapy in patients with inflammatory bowel disease (IBD) remains controversial, especially in the developing world, where its long-term use is restrained by side effects and prohibitive cost. Present study evaluated the relapse rate and its predictors following anti-TNF withdrawal in a cohort of IBD patients from northern India.
Methods
Patients with IBD who received anti-TNF therapy (induction and beyond), and were under follow-up at All India Institute of Medical Sciences, New Delhi, from January 2005 to July 2018 were included. Demographic features, disease characteristics, duration, response to anti-TNF therapy, and relapse rate after its withdrawal were analyzed.
Results
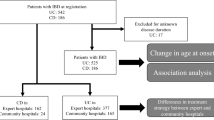
Among 4600 patients with IBD under follow-up, 90 (1.9%) received anti-TNF therapy, of whom 11 were excluded (8—complete records unavailable; 3—received only single dose). Of 79 patients (mean age—40.1 ± 14.2 years; 53.2% males; 31 [39.2%] ulcerative colitis, 47 [59.5%] Crohn’s disease; median follow-up—24 [12–39] months), 9 (11.4%) were primary non-responders, 19 (24.1%) had secondary loss of response, and 51 (64.5%) maintained clinical response on anti-TNF. Anti-TNF was withdrawn in 45 (57%) patients (major causes: financial burden—16.5%; tubercular reactivation—12.7%), of whom 33 were in clinical remission. Over a median follow-up of 26 (7.5–45) months, 15 patients (45.5%) relapsed. Most of them responded to antibiotics, steroids, or anti-TNF agents; only 3 required surgery. On Kaplan-Meier analysis, long disease duration prior to therapy was a significant predictor of relapse (hazard ratio [HR] = 1.33, p = 0.034).
Conclusion
Almost 50% patients with IBD in clinical remission relapse within a year of anti-TNF withdrawal. However, most of these patients have a favorable disease course and respond to medical therapy.


Similar content being viewed by others
References
Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14:111–9.
Singh P, Ananthakrishnan A, Ahuja V. Pivot to Asia: inflammatory bowel disease burden. Intest Res. 2017;15:138–41.
Billiet T, Rutgeerts P, Ferrante M, Van Assche G, Vermeire S. Targeting TNF-α for the treatment of inflammatory bowel disease. Expert Opin Biol Ther. 2014;14:75–101.
Van der Valk ME, Mangen MJ, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNF alpha therapy: results from the COIN study. Gut. 2014;63:72–9.
D'Haens GR, Panaccione R, Higgins PD, et al. The London Position Statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–212.
Puri AS, Desai D, Sood A, Sachdeva S. Infliximab-induced tuberculosis in patients with UC: experience from India-a country with high prevalence of tuberculosis. J Gastroenterol Hepatol. 2017;32:1191–4.
Agarwal A, Kedia S, Jain S, et al. High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India. Intest Res. 2018;16(4):588–98.
Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70.
Bortlik M, Duricova D, Machkova N, et al. Discontinuation of antitumor necrosis factor therapy in inflammatory bowel disease patients: a prospective observation. Scand J Gastroenterol. 2016;51:196–202.
Amiot A, Hulin A, Belhassan M, et al. Therapeutic drug monitoring is predictive of loss of response after de-escalation of infliximab therapy in patients with inflammatory bowel disease in clinical remission. Clin Res Hepatol Gastroenterol. 2015;40:90–8.
Lucidarme C, Petitcollin A, Brochard C, et al. Predictors of relapse following infliximab de-escalation in patients with inflammatory bowel disease: the value of a strategy based on therapeutic drug monitoring. Aliment Pharmacol Ther. 2019;49:147–54.
Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–90.
Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010;4:7–27.
Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32.
Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–44.
Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A.
Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512–30.
Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341–53.
Makharia GK, Ramakrishna BS, Abraham P, et al. Survey of inflammatory bowel diseases in India. Indian J Gastroenterol. 2012;31:299–306.
Kamat N, Kedia S, Ghoshal UC, et al. Effectiveness and safety of adalimumab biosimilar in inflammatory bowel disease: a multicenter study. Indian J Gastroenterol. 2019;38:44–54
Sood A, Midha V, Sharma S, et al. Infliximab in patients with severe steroid-refractory ulcerative colitis: Indian experience. Indian J Gastroenterol. 2014;33:31–4.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9.
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76.
Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–38.
Sandborn WJ, Abreu MT, D’Haens G, et al. Certolizumab pegol in patients with moderate to severe Crohn’s disease and secondary failure to infliximab. Clin Gastroenterol Hepatol. 2010;8:688–95.
Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987–95.
Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–7.
Casanova MJ, Chaparro M, Garcia-Sanchez V, et al. Evolution after anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol. 2017;112:120–31.
Papamichael K, Vande Casteele N, Gils A, et al. Long-term outcome of patients with Crohn’s disease who discontinued infliximab therapy upon clinical remission. Clin Gastroenterol Hepatol. 2015;13:1103–10.
Reenaers C, Mary JY, Nachury M, et al. Long-term outcome after infliximab withdrawal for sustained remission in Crohn’s disease. Gastroenterology. 2016;150:S72.
Gisbert JP, Martın AC, Chaparro M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment Pharmacol Ther. 2015;42:391–405.
Amiot A, Hulin A, Belhassan M, et al. Therapeutic drug monitoring is predictive of loss of response after de-escalation of infliximab therapy in patients with inflammatory bowel disease in clinical remission. Clin Res Hepatol Gastroenterol. 2016;40:90–8.
Paul S, Roblin X, Peyrin-Biroulet L. Letter: infliximab de-escalation based on trough levels in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42:939–40.
Acknowledgments
This study was part of a project under Indian Council of Medical Research-Centre for Advanced Research in Intestinal diseases.
Author information
Authors and Affiliations
Contributions
Conceptualization: Vineet Ahuja, Saurabh Kedia. Methodology: Vineet Ahuja, Saurabh Kedia, Govind Makharia. Formal analysis: Saurabh Kedia, Pabitra Sahu. Funding acquisition: None. Project administration: Vineet Ahuja. Visualization: Vineet Ahuja, Saurabh Kedia. Writing–Pabitra Sahu, Saurabh Kedia, Vineet Ahuja; Writing—review and editing: Vineet Ahuja, Saurabh Kedia, Raju Sharma, Prasenjit Das, Ashish Agarwal, Saransh Jain, Sudheer K Vuyyuru, Bhaskar Kante, Sawan Bopanna. Approval of final manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
PS, SKV, BK, AA, RS, PD, RP, SJ, SB, GM, SK, and VA declare that they have no conflict of interest.
Ethics statement
The study was performed conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research done in: Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi 110 029, India.
Rights and permissions
About this article
Cite this article
Sahu, P., Vuyyuru, S.K., Kante, B. et al. Relapse rate following withdrawal of anti-TNF therapy in patients with inflammatory bowel disease: A real-life cohort from northern India. Indian J Gastroenterol 39, 388–397 (2020). https://doi.org/10.1007/s12664-020-01043-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-020-01043-w