Abstract
Background
The safety and efficacy of sofosbuvir-based treatment (sofosbuvir and ribavirin with or without pegylated interferon-α) for hepatitis C virus (HCV) infection has been established in clinical trials. However, there is limited data regarding safety and efficacy of sofosbuvir-based treatment for HCV infection in a “real-life” cohort. We describe our experience with sofosbuvir-based treatment for HCV infection in a real-life cohort.
Methods
This was a prospective, nonrandomized and observational study at a tertiary care centre in Surat, India. The primary end-point was proportion of the study patients who achieved a sustained virological response 12 weeks after cessation of treatment (SVR 12). Secondary end-points of the study include SVR 4, virological relapse and appearance of adverse events.
Results
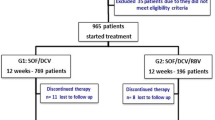
A total of 107 patients with chronic HCV who received sofosbuvir-based treatment were included in the study. During study period, two patients died due to severity of liver complications. Hence, overall rate of SVR 4 and SVR 12 was 98.1 % (n = 103/105) and 94.3 % (n = 99/105), respectively. Among 67 patients with HCV genotype-3 infection, the SVR 12 rate was 92.5 % (n = 62/67), and among 38 patients with HCV genotype-1 infection, the rate of SVR 12 was 97.4 % (n=37/38). A total of 32 (29.9 %) patients reported adverse events during the course of sofosbuvir-based treatment. None of the patient discontinued treatment due to adverse event.
Conclusions
Sofosbuvir-based treatment is safe and efficacious in clinical practice in Indian patients with HCV genotype-1 and genotype-3 infection.
Similar content being viewed by others
References
Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–42.
Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection: Updated Version. Geneva: World Health Organization 2016.
Cornberg M, Deterding K, Manns MP. Present and future therapy for hepatitis C virus. Expert Rev Anti Infect Ther. 2006;4:781–93.
Mangia A, Piazzolla V. Overall efficacy and safety results of sofosbuvir-based therapies in phase II and III studies. Dig Liver Dis. 2014;46 Suppl 5 :S179–85.
Pol S, Sulkowski MS, Hassanein T, et al. Sofosbuvir plus pegylated interferon and ribavirin in patients with genotype 1 hepatitis C virus in whom previous therapy with direct-acting antivirals has failed. Hepatology. 2015;62:129–34.
Ferguson MC. Sofosbuvir with ribavirin is safe and effective in hepatitis C genotype 1 with unfavourable pretreatment characteristics. Evid Based Med. 2014;19:90.
Saxena V, Nyberg L, Pauly M, et al. Safety and efficacy of simeprevir/sofosbuvir in hepatitis C–infected patients with compensated and decompensated cirrhosis. Hepatology. 2015;62:715–25.
Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–57.
Puri P, Anand AC, Saraswat VA, et al. Consensus statement of HCV Task Force of the Indian National Association for Study of the Liver (INASL). Part II: INASL recommendations for management of HCV in India. J Clin Exp Hepatol. 2014;4:117–40.
Gupta V, Kumar A, Sharma P, et al. Sustained virological response rates to antiviral therapy in genotype 1 and 3 chronic hepatitis C patients: a study from north India. J Clin Exp Hepatol. 2014;4:287–92.
Sood A, Midha V, Sood N, Bansal M. Pegylated interferon alfa 2b and oral ribavirin in patients with HCV-related cirrhosis. Indian J Gastroenterol. 2006;25:283–5.
Chakravarti A, Verma V. Distribution of hepatitis C virus genotypes in beta-thalassaemic patients from Northern India. Transfus Med. 2006;16:433–8.
Hissar SS, Goyal A, Kumar M, et al. Hepatitis C virus genotype 3 predominates in North and Central India and is associated with significant histopathologic liver disease. J Med Virol. 2006;78:452–8.
Christdas J, Sivakumar J, David J, Daniel H, Raghuraman S, Abraham P. Genotypes of hepatitis C virus in the Indian sub-continent: a decade-long experience from a tertiary care hospital in South India. Indian J Med Microb. 2013;31:349–53.
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87.
Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–77.
Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993–2001.
Cho Y, Cho EJ, Lee JH, Yu SJ, Yoon JH, Kim YJ. Sofosbuvir-based therapy for patients with chronic hepatitis C: early experience of its efficacy and safety in Korea. Clin Mol Hepatol. 2015;21:358–64.
Steinebrunner N, Sprinzl MF, Zimmermann T, et al. Early virological response may predict treatment response in sofosbuvir-based combination therapy of chronic hepatitis c in a multi-center “real-life” cohort. BMC Gastroenterol. 2015;15:1.
Wehmeyer MH, Jordan S, Lüth S, et al. Efficacy and safety of sofosbuvir-based triple therapy in hepatitis C genotype 4 infection. Dig Liver Dis. 2015;47:811–4.
Tong MJ, Chang PW, Huynh TT, Rosinski AA, Tong LT. Adverse events associated with ribavirin in sofosbuvir-based therapies for patients with chronic hepatitis C: a community practice experience. J Dig Dis. 2016;17:113-21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RM, MK, SN, RT, PN, MS, and VB declare that they have no conflict of interest.
Ethical consideration
The Institutional Review Board has given approval. This study was conducted in accordance with the principles of the Declaration of Helsinki as revised in 2000.
Source of support
None.
Rights and permissions
About this article
Cite this article
Mehta, R., Kabrawala, M., Nandwani, S. et al. Efficacy and safety of sofosbuvir-based therapy for chronic hepatitis C infection in “real-life” cohort. Indian J Gastroenterol 35, 459–464 (2016). https://doi.org/10.1007/s12664-016-0713-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-016-0713-5