Abstract
Purpose
Anabasis articulata (Forssk.) Moq. (Chenopodiaceae), also called Eshnan, Ajremor Berry bearing glasswort, is widely distributed in Syrian, Algerian, Jordan, Lebanon, Saudi Arabia, Egyptian and Iraqi desert, Spain (Alicante, Almería, Granada and Murcia provinces), Mauritania, Western Sahara and Morocco. Anabasis articulata is broadly used in folk medicine to treat diabetes, fever, eczema and kidney infections. The objective of this work was to examine the potential bioactivity of the plant, in vitro and in vivo experiment.
Methods
The sterol-rich extract was identified by GC/MS analysis. The antioxidant potential, anti-tyrosinase and antiproliferative activities, was evaluated by in vitro assays and anti-inflammatory function was determined by in vivo assay.
Results
The results revealed that the chromatographic analysis showed the presence of four sterols in the plant samples. A preliminary evaluation of the antiproliferative effect of A. articulata against two human tumor cell lines, MCF7 and MDA-MB 231, was evaluated by MTT assay. The plant extract was also subjected to four different in vitro antioxidant assays (i.e. total antioxidant capacity, reducing power, DPPH and β-carotene-linoleic acid bleaching). The sterol-rich extract also showed a high anti-tyrosinase activity, and an acetylcholinesterase inhibitory effect, and in vivo toxicological and anti-inflammatory function of A. articulata sterols was tested on rats with carrageenan-induced inflammatory paw edema.
Conclusions
All this evidence suggests the possible application of the aerial part of A. articulata as food additive, in pharmaceutical and cosmetic industries.
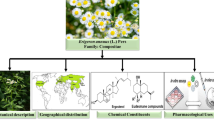
Graphical Abstract








Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Eddouks, M., Maghrani, M., Lemhadri, A., Ouahidi, M.L., Jouad, H.: Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the southeast region of Morocco (Tafilalet). J. Ethnopharmacol. 82(2–3), 97–103 (2002)
Ozenda, P.: Flore et végétation du Sahara. 3ème édition, CNRS Editions, Paris (2004)
Hammiche, V., Maiza, K.: Traditional medicine in Central: 89–94. Sahara: pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 105(3), 358–67 (2006)
Kambouche, N., Merah, B., Derdour, A., Bellahouel, S., Bouayed, J., Dicko, A., Younos, C., Soulimani, R.: Hypoglycemic and antihyperglycemic effects of Anabasis articulata (Forssk) Moq (Chenopodiaceae), an Algerian medicinal plant. Afr. J. Biotechnol. 8(20), 5589–5594 (2009). https://doi.org/10.3732/ajb.0800079
Begley, S.: Beyond vitamins. Newsweek 123, 45–49 (1994)
Benveniste, P.: Sterol biosynthesis. Annu. Rev. Plant Physiol. 37, 25–308 (1986)
Turhan, B., Tayfu, N.P., Deniz, T., Otto, S., Hsan, Ç.: Triterpene saponins from Scabiosa rotata. Phytochemistry 48(5), 867–873 (1997)
Prieto, P., Pineda, M., Aguilar, M.: Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269(2), 337–341 (1999). https://doi.org/10.1006/abio.1999.4019
Oyaizu, M.: Studies on products of browning reaction prepared from glucose amine. Jpn. J. Nutr. 44(6), 307–315 (1986). https://doi.org/10.5264/eiyogakuzashi.44.307
Sanchez-Moreno, C., Larrauri, J.A., Saura-Calixto, F.: A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 76(2), 270–276 (1998)
Moure, A., Franco, D., Sineiro, J., Dominguez, H., Nunez, M.J., Lema, G.M.: Evaluation of extracts from Gevuina avellana hulls as antioxidants. J. Agric. Food Chem. 48(9), 3890–3897 (2000)
Chang, T.S.: An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 10(6), 2440–2475 (2009). https://doi.org/10.3390/ijms10062440
Mroczek, T.: Highly efficient, selective and sensitive molecular screening of acetylcholinesterase inhibitors of natural origin by solid-phase extraction-liquid chromatography/electrospray ionisation-octopole-orthogonal acceleration time-of-flight-mass spectrometry and novel thinlayer chromatography-based bioautography. J. Chromatogr. A 1216(12), 2519–2528 (2009)
Ellman, G.L., Courtney, K.D., Andres, V., Featherstone, R.M.: A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7(2), 88–95 (1961)
Ingkaninan, K., de Best, C.M., van der Heijden, R., Hofte, A.J.P., Karabatak, B., Irth, H., Tjaden, U.R., van der Greef, J., Verpoorte, R.: High-performance liquid chromatography with on-line coupled UV, mass spectrometric and biochemical detection for identification of acetylcholinesterase inhibitors from natural products. J. Chromatogr. A 872(1–2), 61–73 (2000)
Trovato, A., Raneri, E., Kouladis, M., Tzakou, O., Taviano, M.F., Galati, E.M.: Anti-inflammatory and analgesic activity of Hypericum empetrifolium Willd. (Guttiferae). Farmaco 56(5–7), 455–457 (2001)
Belyagoubi-Benhammou, N., Belyagoubi, L., Gismondi, A., Di Marco, G., Canini, A., Atik-Bekkara, F.: GC/MS analysis, and antioxidant and antimicrobial activities of alkaloids extracted by polar and apolar solvents from the stems of Anabasis articulata. Med. Chem. Res. 28, 754–767 (2019). https://doi.org/10.1007/s00044-019-02332-6
El Dine, R.S., Abdallah, H.M., Kandil, Z.A., Zaki, A., Khan, S., Khan, A.: PPARα and γ activation effects of new nor-triterpenoidal saponins from the aerial parts of Anabasis articulata. Planta Med. 85(4), 274–281 (2018)
Garcia-Llatas, G., Rodriguez-Estrada, M.T.: Current and new insights on phytosterol oxides in plant sterol-enriched food. Chem. Phys. Lipids 164(6), 607–624 (2011)
Bouic, P., Clark, A., Lamprecht, J., Freestone, M., Pool, E., Liebenberg, R., Kotze, D., Van Jaarsveld, P.: The effects of B-sitosterol (BSS) and B-sitosterol glucoside (BSSG) mixture on selected immune parameters of marathon runners: inhibition of post marathon immune suppression and inflammation. Int. J. Sports Med. 20(4), 258–62 (1999)
Awad, A., Chinnam, M., Fink, C., Bradford, P.: β-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine 14(11), 747–754 (2007)
Kim, Y.S., Li, X.F., Kang, K.H., Ryu, B., Kim, S.K.: Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 47(8), 433–438 (2014)
Ali, H., Dixit, S., Ali, D., Alqahtani, S.M., Alkahtani, S., Alarifi, S.: Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des. Dev. Ther. 9, 2793–2800 (2015)
Ayaz, M., Sadiq, A., Wadood, A., Junaid, M., Ullah, F., Zaman Khan, N.: Cytotoxicity and molecular docking studies on phytosterols isolated from Polygonum hydropiper L. Steroids 141, 30–35 (2019). https://doi.org/10.1016/j.steroids.2018.11.005
Metwally, N.S., Mohamed, A.M., ELSharabasy, F.S.: Chemical constituents of the Egyptian plant Anabasis articulata (Forssk) Moq and its antidiabetic effects on rats with streptozotocin-induced diabetic hepatopathy. J. Appl. Pharm. Sci. 2(4), 54–65 (2012)
Chai, J.W., Kuppusamy, U.R., Kanthimathi, M.S.: Beta-sitosterol induces apoptosis in MCF-7 cells. Malays. J. Biochem. Mol. Biol. 16(2), 28–30 (2008)
Saeidnia, S., Manayi, A., Ahmad, R., Gohari-Abdollahi, M.: The story of β-sitosterol—a review. Eur. J. Med. Plant 4(5), 590–609 (2014)
Park, C., Moon, D.O., Rhu, C.H., Choi, B.T., Lee, W.H., Kim, G.Y., Choi, Y.H.: β-Sitosterol induces antiproliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biol. Pharm. Bull. 30(7), 1317–1323 (2007)
Ju, Y.H., Clausen, L.M., Allred, K.F., Almada, A.L., Helferich, W.G.: β-Sitosterol, β-sitosterol glucoside, and a mixture of β-sitosterol and β-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovariectomized athymic mice. J. Nutr. 134(5), 1145–1151 (2004)
Awad, A., Downie, A.C., Fink, C.S.: Inhibition of growth and stimulation of apoptosis by beta-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int. J. Mol. Med. 5(5), 541–545 (2000). https://doi.org/10.3892/ijmm.5.5.541
Manayi, A., Saeidnia, S., Ostad, S.N., Hadjiakhoondi, A., Shams Ardekani, M.R., Vazirian, M., Akhtar, Y., Khanavi, M.: Chemical constituents and cytotoxic effect of the main compounds of Lythrum salicaria L. Z. Naturforsch. 68(9–10), 367–375 (2013)
Gupta, R., Sharma, A.K., Dobhal, M., Sharma, M., Gupta, R.: Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 3(1), 29–37 (2011)
Baskar, A.A., Al Numair, K.S., Paulraj, M.G., Alsaif, M.A., Al Muamar, M., Ignacimuthu, S.: β-Sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J. Med. Food 15(4), 335–343 (2012). https://doi.org/10.1089/jmf.2011.1780
Vivancos, M., Moreno, J.J.: β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic. Biol. Med. 39(1), 91–7 (2005)
Yoshida, Y., Niki, E.: Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 49, 277–280 (2003)
Bozin, B., Mimica-Dukic, N., Samojlik, I., Goran, A., Igic, R.: Phenolic as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 111(4), 925–929 (2008)
Senhaji, S., Lamchouri, F., Toufik, H.: Phytochemical content, antibacterial and antioxidant potential of endemic plant Anabasis aretioïdes Coss. & Moq. (Chenopodiaceae). Biomed. Res. Int. 2020(6), 1–16 (2020)
El-Haci, I., Atik-Bekkara, F., Mazari, W., Gherib, M.: Phenolics content and antioxidant activity of some organic extracts of endemic medicinal plant Anabasis aretioides Coss. & Moq. from Algerian Sahara. Pharmacogn. J. 5(3), 108–112 (2013)
Shi, C., Wu, F., Zhu, X., Xu, J.: Incorporation of β-sitosterol into the membrane increases resistance to oxidative stress and lipid peroxidation via estrogen receptor mediated PI3K/GSK3β signaling. Biochim. Biophys. Acta 1830(3), 2538–2544 (2013)
Abdallah, H.M., Abdel-Naim, A.B., Ashour, O.M., Shehata, I.A., Abdel-Sattar, E.A.: Anti-inflammatory activity of selected plants from Saudi Arabia. Z. Naturforsch. C. J. Biosci. 69(1–2), 1–9 (2014)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ben Menni, D., Belyagoubi-Benhammou, N., Benmahieddine, A. et al. Identification of Sterols from Anabasis articulata (Forssk.) Moq. (Chenopodiaceae) Growing in Algeria and Study of Their Potential Bioactivity. Waste Biomass Valor 13, 3283–3295 (2022). https://doi.org/10.1007/s12649-022-01717-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01717-w