Abstract
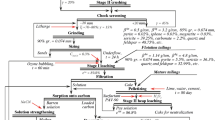
Serpentinite tailings from La Nationale chrysotile mine in Thetford Mines (Quebec, Canada) were studied to extract the Mg contained therein. The study began with an initial chemical characterization of the residue to determine the Mg concentration in the different grain size fractions. The resulting Mg-containing fractions were leached under a variety of parameters such as type of acid, acid concentration, treatment time, and temperature, and the obtained solution was neutralized with NaOH for the selective recovery of the metals. The results of this study were used to design a process to obtain Mg as a marketable chemical. The tested process consists of a leaching step using an H2SO4 solution followed by the purification of the leachates using NaOH at pH 8 and Mg recovery with NaOH at pH 10. A final product composed of mirabilite (Na2SO4) and brucite (Mg(OH)2) was obtained. The procedure was tested, and its economic viability was discussed. The approach proved to be technically feasible, promoting clean production.




Similar content being viewed by others
References
Aïtcin, P.C., Delvaux, P.: L’amiante Chrysotile. Université de Sherbrooke, Sherbrooke (1978)
American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF): Standard Methods for the Examination of Water and Wastewater, 20th edn. APHA, Washington, DC (1999)
Apostolidis, C.I., Distin, P.A.: The kinetics of the sulphuric acid leaching of nickel and magnesium from reduction roasted serpentine. Hydrometallurgy 3, 181–196 (1978)
Bates, T.F., Comer, J.J.: Further observations on the morphology of chrysotile and halloysite1. Clays Clay Miner. 6, 237–248 (1957)
Brookins, D.G.: Eh-pH Diagrams for Geochemistry. Springer, Berlin (1988)
Brown, R.E.: Magnola: The Noranda magnesium process. Light Metal Age 56(1/2), 60–63 (1998)
Cecchi, E.:. Revalorisation des résidus d’extraction d’amiante blanc par la production de chlorure de magnésium via la réaction de carbochloruration. Ph.D. Thesis, INRS-ETE, Université du Québec, Québec (2008)
Claassen, J.O., Meyer, E.H.O., Rennie, J., Sandenbergh, R.F.: Iron precipitation from zinc-rich solutions: defining the Zincor Process. Hydrometallurgy 67(1), 87–108 (2002)
Dutrizac, J.E., Chen, T.T., White, C.W.: Fundamentals of serpentine leaching in hydrochloric acid media. In: Kaplan, H.I., Hryn, J.N., Clow, B.B. (eds.) Magnesium Technology. TMS Annual Meeting, The Minerals, Metals and Materials Society, Nashville, pp. 41–51 (2000)
El-Leef, E.S.M.A., Abeidu, A.E.M., Mahdy, A.E.M.: Utilization of serpentine ore for production of magnesium sulphate. World J. Eng. Pure Appl. Sci. 2(2), 31–39 (2012)
Fedoročková, A., Hreus, M., Raschman, P., Sučik, G.: Dissolution of magnesium from calcined serpentinite in hydrochloric acid. Miner. Eng. 32, 1–4 (2012)
Fouda, M.F.R., Amin, R.E.-S., Abd-Elzaher, M.M.: Extraction of magnesia from Egyptian serpentine ore via reaction with different acids. I. Reaction with sulfuric acid. Bull. Chem. Soc. Jpn. 69(7), 1907–1912 (1996)
Friedrich H.E., Mordike B.L. (2006) Magnesium Technology-Metallurgy, Design Data, Applications. Springer, Berlin
Gladikova, L., Teterin, V., Freidlina, R.: Production of magnesium oxide from solutions formed by acid processing of serpentinite. Russ. J. Appl. Chem. 81(5), 889–891 (2008)
Habashi, F.: Textbook of Hydrometallurgy, 2nd edn. Département des mines, métallurgies et génie des matériaux, Université Laval, Sainte-Foy (1999)
Habashi, F.: Magnesium. Handbook of Extractive Metallurgy. Wiley, Weinheim (1997)
Harben, P.W., Smith, C. Jr: Industrial minerals and rocks; commodities, markets and uses. SME, Littleton, pp. 679–683 (2006)
Karidakis, T., Agatzini-Leonardou, S., Neou-Syngouna, P.: Removal of magnesium from nickel laterite leach liquors by chemical precipitation using calcium hydroxide and the potential use of the precipitate as a filler material. Hydrometallurgy 76, 105–114 (2005)
Kim, J.: Magnesium Extraction from Asbestos Mine Tailings: A Report. Howard Manosh Vermont Asbestos Group, Vermont Geological Survey, Department of Environmental Conservation, Waterbury (1998)
Lackner, K.S., Wendt, C.H., Butt, D.P., Joyce, B.L., Sharp, D.H.: Carbon dioxide disposal in carbonate minerals. Energy. 20, 1153–1170 (1995)
Levenspiel, O.: Chemical Reaction Engineering. Wiley, New York (1978)
Luce, R. W., Bartlett, R. W., Parks, G. A.: Dissolution kinetics of magnesium silicates. Geochim. Et Cosmochim. Acta. 36(1), 35–50 (1972)
Monhemius, A. J.: Precipitation diagrams for metal hydroxides, sulfates, arsenates and phosphates. T. I. Min. Metall. C 86, C202–C206 (1977)
Mossman, B.T., Bignon, J., Corn, M., Seaton, A., Gee, J.B.L.: Asbestos: scientific developments and implications for public policy. Science 247(4940), 294–301 (1990)
Nagamori, M., Boivin, J.A.: Technico-economic simulation for the HCl-leaching of hybrid serpentine and magnesite feeds. Can. Metall. Q. 40(1), 47–60 (2001)
Nduagu, E., Björklöf, T., Fagerlund, J., Mäkilä, E., Salonen, J., Geerlings, H., Zevenhoven, R.: Production of magnesium hydroxide from magnesium silicate for the purpose of CO2 mineralization—Part 2: Mg extraction modeling and application to different Mg silicate rocks. Miner. Eng. 30, 87–94 (2012). doi:10.1016/j.mineng.2011.12.002
Pasquier, L.-C., Mercier, G., Blais, J.-F., Cecchi, E., Kentish, S.: Reaction mechanism for the aqueous-phase mineral carbonation of heat-activated serpentine at low temperatures and pressures in flue gas conditions. Environ. Sci. Technol. 48, 5163–5170 (2014)
Petrovski, P., Gligoric, M.: Usage of serpentine for MgO and active SiO2 production. In: Vincenzini, P.. (ed.), High Tech Ceramics. Elsevier, Amsterdam, pp. 2267–2278 (1987)
Pigg, R.: Uses of chrysotile asbestos. Ann. Occup. Hyg. 38(4), 453–458 (1994)
Pourbaix, M.: Atlas of Electrochemical Equilibria in Aqueous Solutions. National Association of Corrosion Engineers, Houston (1974)
Ramachandra Rao, S.: Resource Recovery and Recycling from Metallurgical wastes. Waste Management Series, vol. 7. Elsevier, Amsterdam (2006)
Ray, M.S., Sneesby, M.G. Chemical Engineering Design Project: A Case Study Approach, 2nd edn. Gordon and Breach Science Publishers, Amsterdam (1998)
Schrier, H.: Asbestos in the Natural ENVIRONMENT. Studies in Environmental Science. No. 37. Elsevier, Amsterdam (1989)
Skinner, H.C.W., Ross, M., Frondel, C.: Asbestos and Other Fibrous Materials. Oxford University Press, New York (1988)
Taubert, L.: Hydrochloric attack of serpentinites: Mg2+ leaching from serpentinites. Magnes. Res. 13(3), 167–173 (2000)
Teir, S., Revitzer, H., Eloneva, S., Fogelholm, C.J., Zevenhoven, R.: Dissolution of natural serpentinite in mineral and organic acids. Int. J. Miner. Process. 83, 36–46 (2007)
U.S. Department of Health and Human Services: 12th Report on Carcinogens. Public Health Service, National Toxicology Program, Research Triangle Park (2011)
United States Environmental Protection Agency (USEPA): Toxicity characteristic leaching procedure, method 1311. http://www.epa.gov/wastes/hazard/testmethods/SW846/pdfs/1311.pdf (1992)
Veblen, D.R., Wylie, A.G.: Mineralogy of amphiboles and 1:1 layer silicates. In: Guthrie, G.D., Mossman, B.T. (eds.) Health Effects of Mineral Dusts. Reviews in Mineralogy, vol. 28, pp. 61–137. Mineralogical Society of America, Bookcrafters Inc., Chelsea (1993)
Wulandari, W., Brooks, G., Rhamdhani, M., Monaghan, B.: Magnesium: current and alternative production routes. The 40th Chemeca: Australasian Conference on Chemical Engineering, September 26–29, Adelaide (2010)
Yoo, K., Kim, B.S., Kim, M.S., Lee, J.C., Jeong, J.: Dissolution of magnesium from serpentine mineral in sulfuric acid solution. Mater. Trans. 50(5), 1225–1230 (2009)
Zhang, Q., Sugyiama, K., Saito, F.: Enhancement of acid extraction of magnesium and silicon from serpentine by mechanochemical treatment. Hydrometallurgy 45(3), 323–331 (1997)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sierra, C., Chouinard, S., Pasquier, L.C. et al. Feasibility Study on the Utilization of Serpentine Residues for Mg(OH)2 Production. Waste Biomass Valor 9, 1921–1933 (2018). https://doi.org/10.1007/s12649-017-9926-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9926-9