Abstract
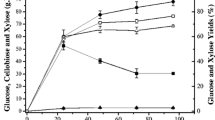
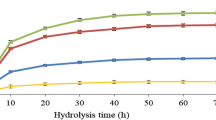
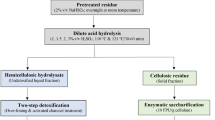
The leaves of sugarcane (Saccharum officinarum) are agricultural wastes that can be converted to bioethanol by separate hydrolysis and fermentation (SHF) processes. The effect of dilute acid hydrolysis conditions such as acid concentration, sugarcane leave concentration and hydrolysis time on sugar production were investigated. The optimized conditions were at 1%v/v of H2SO4, 100 g/L of sugarcane leaves, and hydrolysis time 60 min. The hydrolysate yielded sugar monomers at a concentration of 14.48 g/L of xylose and 2.59 g/L of glucose that was neutralized prior to fermentation. The fermentation process was carried out using shaken and unshaken stages. The shaken stage was maintained at 30 °C at 150 rpm for 24 h. It was found that Pichia stipitis BCC 15191 consumed only glucose then xylose after glucose depletion, while Candida shehatae TISTR 5843 used the both sugars concurrently in the exponential phase. Aggregation of P. stipitis BCC 15191 cells occurred during the stationary phase. Maximal ethanol yields of 0.21 and 0.20 g Ethanol/g Sugar consumptions were obtained for C. shehatae TISTR 5843 and P. stipitis BCC 15191, respectively. This study demonstrates the potential value of this agricultural waste as a useful feedstock for biological generation of bioethanol.






Similar content being viewed by others
References
Plangklang, P., Reungsang, A., Pattra, S.: Enhanced bio-hydrogen production from sugarcane juice by immobilized Clostridium butyricum on sugarcane bagasse. Int. J. Hydrog. Energy 37(20), 15525–15532 (2012). doi:10.1016/j.ijhydene.2012.02.186
Jones, D.L.: Potential air emission impacts of cellulosic ethanol production at seven demonstration refineries in the United States. J. Air & Waste Manag. Assoc. 60(9), 1118–1143 (2010)
Liu, G., Liao, Y., Guo, S., Ma, X., Zeng, C., Wu, J.: Thermal behavior and kinetics of municipal solid waste during pyrolysis and combustion process. Appl. Therm. Eng. 98, 400–408 (2016). doi:10.1016/j.applthermaleng.2015.12.067
Moodley, P., Kana, E.B.G.: Optimization of xylose and glucose production from sugarcane leaves (Saccharum officinarum) using hybrid pretreatment techniques and assessment for hydrogen generation at semi-pilot scale. Int. J. Hydrog. Energy 40(10), 3859–3867 (2015). doi:10.1016/j.ijhydene.2015.01.087
Gonçalves, G.d.C., Pereira, N.C., Veit, M.T.: Production of bio-oil and activated carbon from sugarcane bagasse and molasses. Biomass Bioenergy. 85, 178–186 (2016). doi:10.1016/j.biombioe.2015.12.013
Hossain, A.K., Serrano, C., Brammer, J.B., Omran, A., Ahmed, F., Smith, D.I., Davies, P.A.: Combustion of fuel blends containing digestate pyrolysis oil in a multi-cylinder compression ignition engine. Fuel 171, 18–28 (2016). doi:10.1016/j.fuel.2015.12.012
Xu, R., Ferrante, L., Briens, C., Berruti, F.: Bio-oil production by flash pyrolysis of sugarcane residues and post treatments of the aqueous phase. J. Anal. Appl. Pyrolysis 91(1), 263–272 (2011). doi:10.1016/j.jaap.2011.03.001
Tang, Y.-Q., An, M.-Z., Zhong, Y.-L., Shigeru, M., Wu, X.-L., Kida, K.: Continuous ethanol fermentation from non-sulfuric acid-washed molasses using traditional stirred tank reactors and the flocculating yeast strain KF-7. J. Biosci. Bioeng. 109(1), 41–46 (2010). doi:10.1016/j.jbiosc.2009.07.002
Koike, Y., An, M.-Z., Tang, Y.-Q., Syo, T., Osaka, N., Morimura, S., Kida, K.: Production of fuel ethanol and methane from garbage by high-efficiency two-stage fermentation process. J. Biosci. Bioeng. 108(6), 508–512 (2009). doi:10.1016/j.jbiosc.2009.06.007
Shahsavarani, H., Hasegawa, D., Yokota, D., Sugiyama, M., Kaneko, Y., Boonchird, C., Harashima, S.: Enhanced bio-ethanol production from cellulosic materials by semi-simultaneous saccharification and fermentation using high temperature resistant Saccharomyces cerevisiae TJ14. J. Biosci. Bioeng. 115(1), 20–23 (2013). doi:10.1016/j.jbiosc.2012.07.018
Schneiderman, S.J., Johnson, R.W., Menkhaus, T.J., Gilcrease, P.C.: Quantifying second generation ethanol inhibition: design of experiments approach and kinetic model development. Bioresour. Technol. 179, 219–226 (2015). doi:10.1016/j.biortech.2014.11.087
Zhang, G.-C., Liu, J.-J., Kong, I.I., Kwak, S., Jin, Y.-S.: Combining C6 and C5 sugar metabolism for enhancing microbial bioconversion. Curr. Opin. Chem. Biol. 29, 49–57 (2015). doi:10.1016/j.cbpa.2015.09.008
Hu, X., Wang, S., Wu, L., Dong, D., Mahmudul Hasan, M., Li, C.-Z.: Acid-treatment of C5 and C6 sugar monomers/oligomers: Insight into their interactions. Fuel Process. Technol. 126, 315–323 (2014). doi:10.1016/j.fuproc.2014.05.024
Li, Y.-C., Li, G.-Y., Gou, M., Xia, Z.-Y., Tang, Y.-Q., Kida, K.: Functional expression of xylose isomerase in flocculating industrial Saccharomyces cerevisiae strain for bioethanol production. J. Biosci. Bioeng. 121(6), 685–691 (2016). doi:10.1016/j.jbiosc.2015.10.013
Kupiainen, L., Ahola, J., Tanskanen, J.: Kinetics of glucose decomposition in formic acid. Chem. Eng. Res. Des. 89(12), 2706–2713 (2011). doi:10.1016/j.cherd.2011.06.005
Huang, Y.-B., Fu, Y.: Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 15(5), 1095–1111 (2013). doi:10.1039/C3GC40136G
Boochapun, S., Lamamorphanth, W., Kamwilaisak, K.: The acid hydrolysis of sugarcane leaves as a biofeedstook for bioethanol production. Adv. Mater. Res. 931–932, 194–199 (2014). doi:10.4028/www.scientific.net/AMR.931-932.194
Jeffries, T.W.: Utilization of xylose by bacteria, yeasts, and fungi. Adv. Biochem. Eng. Biotechnol. 27, 1–32 (1983)
Cavka, A., Jo¨nsson, L.J.: Comparison of the growth of filamentous fungi and yeasts in lignocellulose-derived media. Biocatal. Agric. Biotechnol. 3(4), 197–204 (2014). doi:10.1016/j.bcab.2014.04.003
Abbi, M., Kuhad, R.C., Singh, A.: Bioconversion of pentose sugars to ethanol by free and immobilized cells of Candida shehatae (NCL-3501): fermentation behaviour. Process Biochem. 31(6), 555–560 (1996). doi:10.1016/S0032-9592(95)00104-2
Delgenes, J.P., Moletta, R., Navarro, J.M.: Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzyme Microb. Technol. 19(3), 220–225 (1996). doi:10.1016/0141-0229(95)00237-5
Zhao, L., Yu, J., Zhang, X., Tan, T.: The ethanol tolerance of pachysolen tannophilus in fermentation on Xylose. Appl. Biochem. Biotechnol. 160(2), 378–385 (2010). doi:10.1007/s12010-008-8308-y
Burdon, R.H., Paul, P.H.V.K., Paul T.S.: Chap. 2: methods of cell counting and assaying cell viability. In: Burdon, R.H., Paul, P.H.v.K., T.S. (eds.) Laboratory techniques in biochemistry and molecular biology, vol. 18. pp. 7–17. Elsevier, Amsterdam (1988)
Van Soest, P.J., Robertson, J.B., Lewis, B.A.: Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74(10), 3583–3597 (1991). doi:10.3168/jds.S0022-0302(91)78551-2
Kobayashi, H., Ito, Y., Komanoya, T., Hosaka, Y., Dhepe, P.L., Kasai, K., Hara, K., Fukuoka, A.: Synthesis of sugar alcohols by hydrolytic hydrogenation of cellulose over supported metal catalysts. Green Chem. 13(2), 326–333 (2011). doi:10.1039/C0GC00666A
Playne, M.J.: Determination of ethanol, volatile fatty acids, lactic and succinic acids in fermentation liquids by gas chromatography. J. Sci. Food Agric. 36(8), 638–644 (1985). doi:10.1002/jsfa.2740360803
Omari, K.W., Besaw, J.E., Kerton, F.M.: Hydrolysis of chitosan to yield levulinic acid and 5-hydroxymethylfurfural in water under microwave irradiation. Green Chem. 14(5), 1480–1487 (2012). doi:10.1039/C2GC35048C
Harmsen, P., Huijgen, W., Bermudez, L., Bakker, R.: Literature review of physical and chemical pretreatment processes for lignocellulosic biomass. Energy Research Centre of the Netherlands, Petten, pp. 10–13 (2010)
Behera, S., Arora, R., Nandhagopal, N., Kumar, S.: Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 36, 91–106 (2014). doi:10.1016/j.rser.2014.04.047
Argun, H., Onaran, G.: Glucose and 5-hydroxymethylfurfural production from cellulosic waste by sequential alkaline and acid hydrolysis. Renew. Energy 96(Part A), 442–449 (2016). doi:10.1016/j.renene.2016.04.082
Riansa-ngawong, W., Prasertsan, P.: Optimization of furfural production from hemicellulose extracted from delignified palm pressed fiber using a two-stage process. Carbohydr. Res. 346(1), 103–110 (2011). doi:10.1016/j.carres.2010.10.009
Antal, M.J. Jr., Leesomboon, T., Mok, W.S., Richards, G.N.: Mechanism of formation of 2-furaldehyde from d-xylose. Carbohydr. Res. 217(C), 71–85 (1991). doi:10.1016/0008-6215(91)84118-X
He, J., Zhang, W., Liu, X., Xu, N., Xiong, P.: Optimization of prehydrolysis time and substrate feeding to improve ethanol production by simultaneous saccharification and fermentation of furfural process residue. J. Biosci. Bioeng. 122(5), 563–569 (2016). doi:10.1016/j.jbiosc.2016.04.012
Robinson, J.M., Burgess, C.E., Bently, M.A., Brasher, C.D., Horne, B.O., Lillard, D.M., Macias, J.M., Mandal, H.D., Mills, S.C., O’Hara, K.D.: The use of catalytic hydrogenation to intercept carbohydrates in a dilute acid hydrolysis of biomass to effect a clean separation from lignin. Biomass Bioenergy 26(5), 473–483 (2004)
Laopaiboon, P., Thani, A., Leelavatcharamas, V., Laopaiboon, L.: Acid hydrolysis of sugarcane bagasse for lactic acid production. Bioresour. Technol. 101(3), 1036–1043 (2010). doi:10.1016/j.biortech.2009.08.091
da Silva, A.S.A., Inoue, H., Endo, T., Yano, S., Bon, E.P.S.: Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 101(19), 7402–7409 (2010). doi:10.1016/j.biortech.2010.05.008
Cheung, S.W., Anderson, B.C.: Ethanol production from wastewater solids, Water Environment and Technology, Medium: X Size pp. 55–60 (1996)
Hilpmann, G., Becher, N., Pahner, F.A., Kusema, B., Mäki-Arvela, P., Lange, R., Murzin, D.Y., Salmi, T.: Acid hydrolysis of xylan. Catal. Today 259, 376–380 (2016). doi:10.1016/j.cattod.2015.04.044
Mäki-Arvela, P., Salmi, T., Holmbom, B., Willför, S., Murzin, D.Y.: Synthesis of sugars by hydrolysis of hemicelluloses- a review. Chem. Rev. 111(9), 5638–5666 (2011). doi:10.1021/cr2000042
Aguilar, R., Ramírez, J.A., Garrote, G., Vázquez, M.: Kinetic study of the acid hydrolysis of sugar cane bagasse. J. Food Eng. 55(4), 309–318 (2002). doi:10.1016/S0260-8774(02)00106-1
Rodríguez-Chong, A., Alberto Ramírez, J., Garrote, G., Vázquez, M.: Hydrolysis of sugar cane bagasse using nitric acid: a kinetic assessment. J. Food Eng. 61(2), 143–152 (2004). doi:10.1016/S0260-8774(03)00080-3
Lin, T.-H., Huang, C.-F., Guo, G.-L., Hwang, W.-S., Huang, S.-L.: Pilot-scale ethanol production from rice straw hydrolysates using xylose-fermenting Pichia stipitis. Bioresour. Technol. 116, 314–319 (2012). doi:10.1016/j.biortech.2012.03.089
Mozes, N., Schinckus, L.L., Ghommidh, C., Navarro, J.-M., Rouxhet, P.G.: Influence of medium composition on surface properties and aggregation of a Saccharomyces cerevisiae strain. Colloids Surf. B. 3(1–2), 63–74 (1994). doi:10.1016/0927-7765(93)01113-6
Nigam, J.N.: Ethanol production from wheat straw hemicellulose hydrolysate by Pichia stipitis. J. Biotechnol. 87(1), 17–27 (2001). doi:10.1016/S0168-1656(00)00385-0
Cheng, C., Zhang, M., Xue, C., Bai, F., Zhao, X.: Development of stress tolerant Saccharomyces cerevisiae strains by metabolic engineering: new aspects from cell flocculation and zinc supplementation. J. Biosci. Bioeng. 123(2), 141–146 (2017). doi:10.1016/j.jbiosc.2016.07.021
Hickert, L.R., da Cunha-Pereira, F., de Souza-Cruz, P.B., Rosa, C.A., Ayub, M.A.Z.: Ethanogenic fermentation of co-cultures of Candida shehatae HM 52.2 and Saccharomyces cerevisiae ICV D254 in synthetic medium and rice hull hydrolysate. Bioresour. Technol. 131, 508–514 (2013). doi:10.1016/j.biortech.2012.12.135
Chandel, A.K., Singh, O.V., Rao, L.V., Chandrasekhar, G., Narasu, M.L.: Bioconversion of novel substrate Saccharum spontaneum, a weedy material, into ethanol by Pichia stipitis NCIM3498. Bioresour. Technol. 102(2), 1709–1714 (2011). doi:10.1016/j.biortech.2010.08.016
Chandel, A.K., Kapoor, R.K., Singh, A., Kuhad, R.C.: Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour. Technol. 98(10), 1947–1950 (2007). doi:10.1016/j.biortech.2006.07.047
Lin, T.-H., Guo, G.-L., Hwang, W.-S., Huang, S.-L.: The addition of hydrolyzed rice straw in xylose fermentation by Pichia stipitis to increase bioethanol production at the pilot-scale. Biomass Bioenergy. 91, 204–209 (2016). doi:10.1016/j.biombioe.2016.05.012
Taherzadeh, M.J., Gustafsson, L., Niklasson, C., Liden, G.: Physiological effects of 5-hydroxymethylfurfural on Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 53(6), 701–708 (2000)
Sanchez, B., Bautista, J.: Effects of furfural and 5-hydroxymethylfurfural on the fermentation of Saccharomyces cerevisiae and biomass production from Candida guilliermondii. Enzym. Microb. Technol. 10(5), 315–318 (1988). doi:10.1016/0141-0229(88)90135-4
Gorke, B., Stulke, J.: Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev Microbiol. 6(8), 613–624 (2008)
Acknowledgements
We gratefully acknowledge Khon Kean University for financial support (600023), Farm Engineering and Automatic Control Technology, Applied Engineering for Important Crops of the Northeast Research Group, and the Graduate School at Khon Kaen University for financial support. We are also grateful to Dr. Malinda Salim and Dr. Stephen Jaffe for great input and suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jutakridsada, P., Saengprachatanarug, K., Kasemsiri, P. et al. Bioconversion of Saccharum officinarum Leaves for Ethanol Production Using Separate Hydrolysis and Fermentation Processes. Waste Biomass Valor 10, 817–825 (2019). https://doi.org/10.1007/s12649-017-0104-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0104-x