Abstract
Purpose
This study investigated the chemical characteristics and anaerobic digestion of Chlorella sp. microalgae cultivated on various anaerobic digestion effluents (ADEs) as a nutrient medium. Chlorella sp. was grown in anaerobically digested effluent of dairy wastewater (DW), municipal wastewater sludge (WS), maize silage and swine slurry, and cattle manure (CM).
Methods
To evaluate the anaerobic biodegradability of harvested biomass, 20-days batch anaerobic digestion experiments were used.
Results
It was found that a nutrient medium directly affected nitrogen concentration in the cultivated biomass, as well as the C/N ratio value which ranged 7.2–12.9. Higher C/N ratio of the Chlorella sp. cultivated on DW and WS significantly enhanced the methane production, which was 241 ± 5.5 mL CH4/g VS and 267 ± 10.9 mL CH4/g VS, respectively. The highest biogas production rate of 61.28 ± 2.7 mL/g VS·d and methane concentration in biogas of 69.7 ± 4.1 % were obtained during the digestion of Chlorella sp. biomass cultivated on WS.
Conclusions
These results proved the applicability of ADEs as a nutrient medium for Chlorella sp. cultivation and the impact of a nutrient source on C/N ratio in harvested biomass, which subsequently affected the biogas/methane yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biogas production via anaerobic digestion (AD) has rapidly developed in recent years [1, 2]. Besides renewable energy, biogas plants also produce large amount of liquid anaerobic digestion effluents (ADEs) which may lead to oversupply of ADEs in a short time. ADEs still have high chemical oxygen demand (COD) and they are rich in nitrogen and phosphorus, which excludes the possibility of these wastewaters discharge directly to the environment. Thus, a low-cost method to treat ADEs is needed. Considering both the characteristics of ADEs and nutritional needs of algae, it seems that ADEs may be a useful source of nutrients and microelements to ensure an intensive growth of microalgae biomass with simultaneous contaminants biodegradation [3–5].
Microalgae cultivation have nowadays gained high attention in the field of renewable energy because of their potential to produce large quantities of biomass, resistance to pollution, less water uptake and land requirement and higher bioenergy yield compared to terrestrial biofuel crops [6, 7]. Microalgae biomass can be converted into many biofuels such as biodiesel from cells lipids, hydrogen derived from photobiological processes, heat form direct combustion and biogas produced during anaerobic digestion [8, 9]. Many studies have recommended AD of microalgae biomass as a profitable solution for biogas generation [10–12]. Recent studies on AD of algal biomass have reported the methane yield of 231 mL CH4/g VS for Navicula occulta, 261 mL CH4/g VS for Scenedesmus sp., 307 mL CH4/g VS for Chlorella vulgaris, 350 mL CH4/g VS for Phaeodactylum tricornutum, 280 mL CH4/g VS for Spirulina platensis [13, 14]. Thus, this is evident that microalgae biomass have good methane potential and its AD can become commercially viable [15].
Coupling microalgae culture and ADEs treatment has been already explored by Cheng et al. [2] using ADE of swine manure, Morales-Amaral et al. [16] using centrate from AD, Yang et al. [17] using anaerobic digested starch wastewater, Park et al. [18] using ADE of livestock waste, Cai et al. [19] using ADE of municipal wastewater, Erkelens et al. [20] using microalgae digestate effluent. However, the effects of ADEs characteristics on microalgal growth and the subsequent AD of obtained biomass are still poorly studied [21, 22].
The current study evaluated the potential suitability of ADEs derived from different sources as a nutrient medium for Chlorella sp. cultivation and the subsequent biogas potential of harvested biomass. The selected ADEs used in the study for Chlorella sp. cultivation were anaerobically digested effluents of dairy wastewater, municipal wastewater sludge, maize silage and swine slurry, and cattle manure.
Materials and Methods
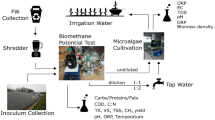
Experimental Design of Microalgae Cultivation
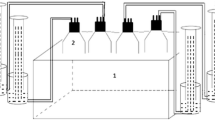
Microalgae biomass cultivation on ADEs was carried out in a laboratory scale with the use of vertical, closed photobioreactors (PBRs) of 7.6 cm diameter and 0.55 m height, with an active volume of 2.5 L. The PBRs were made of transparent glass. A 18 W cool-white fluorescent light (700 lx, Osram, Germany) with reflector was used as a constant light source. The supply of CO2 ensured by continuous inflow of air (at 250 L/h), providing the appropriate mixing of the cultivation medium and homogeneity of conditions in the entire PBR’s volume. The temperature of the culture was 22.0 ± 2.0 °C. Proper thermal conditions were continuously monitored by temperature sensors inside PBRs.
Microalgae Inoculum and Cultivation
Algae of the genus Chlorella sp. (BA0103) used in the experiment originated from the Culture Collection of Baltic Algae Institute of Oceanography, University of Gdańsk, Poland. In all experimental variants, the initial algae biomass concentration in PBRs reached 250 ± 22 mg total solids (TS)/L.
Experimental variants differed in ADE used as a nutrient medium: variant I—anaerobically digested effluent of dairy wastewater (DW), variant II—anaerobically digested effluent of municipal wastewater sludge (WS), variant III—anaerobically digested effluents of maize silage and swine slurry (MS), and variant IV—anaerobically digested effluents of cattle manure (CM). Characteristics of the ADEs were shown in Table 1.
Before feeding to PBRs, the ADEs were centrifuged at 5000 rpm for 10 min (MPW-251, Donserv, Poland) and then autoclaved at 90 °C for 30 min to remove solid suspensions, obtain supernatant containing substances in the dissolved phase and hygienize the nutrient medium.
The criterion deciding about the applied ADEs volume was the initial total ammonia nitrogen (TAN) concentration in reactors used to grow Chlorella sp., which was set at 160 mg N-NH4/dm3. In order to ensure the desired initial concentration of TAN and equal initial concentration of algae biomass, in all experimental variants the PBRs were fed with 1.54 L of Chlorella sp. biomass (with biomass concentration at 405 ± 31 mg TS/L) and filled up to the level of 2.5 L with ADE and deionized water. Inlet parameters of the cultivation medium were shown in Table 2.
The Chlorella sp. microalgae was grown until the biomass concentration in PBRs ca. 2000 mg TS/L. After a completed cultivation process, algae biomass was concentrated, separated and dehydrated in a sedimentation process and then through centrifugation (MPW-251, Donserv, Poland). Finally, it was subjected to chemical analysis and used as a substrate in fermentative biogas production.
Biogas/Methane Production from Chlorella sp. Microalgae Biomass
Algae biomass fermentation was conducted using respirometers (WTW, Germany) that consisted of reaction tanks with an active volume of 0.5 L coupled tightly with measuring devices recorded an increase of the partial pressure induced by biogas production. Pressure in the reaction tank was recorded every 24 h. The ideal gas equation was the basis for computing the volume of produced biogas in the respirometric tests. The volumes of biogas generated per normal conditions were computed on the basis of pressure changes inside the bottle headspace. Respirometric tests also provided grounds to determine the volumetric biogas production rate (VBPR), depending on the employed experimental variants. Reaction rate constants (k) were determined on the basis of obtained experimental data with the nonlinear regression method using the Statistica 10.0 PL (Statsoft, Inc.) application. A conformity index φ2 was accepted as a measure of curve matching to experimental data, which enabled the reaction order and reaction rate constant k to be determined.
0.5 L reactors were filled with 200 mL anaerobic sludge originated from the closed fermentation tanks of a local municipal wastewater treatment plant. The concentration of volatile solids (VS) seeded into the reactor was 69.2 ± 2.8 % TS. In order to ensure anaerobic conditions inside the respirometers, they were blown through with nitrogen to remove atmospheric air at the beginning of the fermentation. The measurements were carried out at a temperature of 38 °C. In all technological variants, the initial load was 5.0 g VS/L. Tests were carried out for a period of 20 days.
The composition of biogas produced in the headspace of reactors was measured every 24 h using a gastight syringe (20 mL injection volume) and a gas chromatograph (GC, 7890A Agilent) equipped with a thermal conductivity detector (TCD). The GC was fitted with the two Hayesep Q columns (80/100 mesh), two molecular sieve columns (60/80 mesh) and Porapak Q column (80/100) operating at a temperature of 70 °C. The temperature of the injection and detector ports were 150 and 250 °C, respectively. Helium and argon were used as the carrier gases at a flow of 15 mL/min.
Analytical Methods
Total nitrogen (TN), total ammonia nitrogen (TAN), total phosphorus (TP), orthophosphate (P-PO4), and chemical oxygen demand (COD) in ADEs and cultivation media were determined using a DR 5000 spectrophotometer with an HT 200 s mineralizer (Hach-Lange, Germany). The gravimetric method was used to determine total solid (TS) and volatile solids (VS) in samples of microalgae biomass and anaerobic sludge. In those samples dried at 105 °C, TC (total carbon), TOC (total organic carbon) and total nitrogen (TN) were determined by elementary particle analyser Flash 2000 (Thermo Scientific, USA). Total phosphorus (TP) in Chlorella sp. biomass was determined spectrophotometrically (DR 2800, Hach-Lange, Germany) using an ammonium metavanadate and molybdate after prior reaction of sample in acid medium. The pH was determined using a pH-meter (1000L, VWR, Germany).
Statistical Methods
Each experimental variant was conducted in three replications (both Chlorella sp. cultivation and anaerobic digestion). The statistical analysis of results was carried out with Statistica 10.0 PL package (Statsoft, Inc.). The hypothesis on distribution of each analyzed variable was verified with a Shapiro–Wilk W-test. One-way analysis of variance (ANOVA) was applied to determine the significance of difference between variables. Variance homogeneity in groups was checked with a Levene’s test, whereas the significance of differences between the analyzed variables was determined with a Tukey RIR test. In all tests, the level of significance was adopted at p = 0.05.
Results and Discussion
Composition Analysis of ADEs
ADEs used in the study contained organic matter, nitrogen and phosphorus which are essential for the growth of microalgae biomass (Table 2). The highest COD concentration in the culture medium of 1270.8 mg/L was noted with DW medium, while the lowest of 312.7 mg/L with WS medium (Table 2). TN, TAN and TP concentrations was on the similar level in all ADEs used as a nutrient medium (Table 2). The pH of ADEs was about of 7.0 (Table 2), which was within the optimal pH range of 6.0–8.0 for the Chlorella genus [2].
The lowest COD to TN ratio (COD/N) of 1.53 was found in WS. In MS and CM, COD/N ratio was slightly above 5, while the value of 7.05 was obtained with DW medium. The N/P ratio estimated was respectively 11.5, 15.1, 14.3, 14.5 in DW, WS, MS and CM. The optimal C/N mass ratio of microalgae is in the range 4–8 [23]. In turn, the N/P ratio of an algal cell is 7 [24]. This suggested that all the nutrient media were limited in phosphorus. Carbon limitation was found in WS. Many data indicate that that nutrient imbalance may limit the growth of microalgae [2, 3, 19]. The growth of Chlorella PY-ZU1was almost doubled through the addition of phosphate, but adding phosphorus at higher concentrations may have an adverse effect due to higher cell osmotic pressure [2].
In all ADEs, the major component of TN was ammonia nitrogen at the concentration of about 160 mg/L. Ammonium is the preferred form of nitrogen for microalgal growth, but ADEs may contain high levels of total ammonia nitrogen (1000–3000 mg/L) which is toxic to microalgae strains at the concentration of above 100 mg/L have [1, 16]. Inhibitory thresholds depend on the microalgal species and cultivation conditions [3, 4]. It has been reported that TAN concentrations of 364 mg/L inhibited the growth of Scenedesmus sp. [25]. By contrast, Park et al. [26] found that the levels of Scenedesmus sp. inhibition were similar when TAN level ranged 200–500 mg/L. Rhodobacter sphaeroides and Chlorella sorokiniana were not inhibited by relatively high ammonia concentrations, while Spirulina platensis was completely inhibited at a TAN level of 400 mg/L [27]. Cai et al. [19] state, that ammonium at concentrations greater than 450 mg/L is often toxic to microalgae. According to Wang et al. [4], Chlorella sp. is highly ammonia nitrogen tolerant.
ADEs may also contain many other components like vitamins, amino-acids which benefited the growth of microalgae, but they also may contain heavy metals which can be toxic to microalgae [2]. However, heavy metals at trace concentrations may stimulate the growth of microalgae. Chlorella sp. is resistant to the action of heavy metals, owing to which it has been used for treatment of industrial wastewaters [28]. Anaerobic digestion effluent of swine and cattle manure, maize silage (MS and CM) or dairy wastewater (DW) contain too low concentrations of heavy metals to cause negative effect on microalgae growth [2]. Only anaerobically digested effluent of municipal wastewater sludge (WS) may contain metallic inhibitors to microalgae growth [16, 29].
Composition of Chlorella sp. Microalgae Cultivated on ADEs
The chemical characteristics of Chlorella sp. biomass depending on the cultivation medium was investigated (Table 3). The harvested algae biomass was mainly composed of organic fraction (84.2–86.8 % TS). Even though carbon limitation was found in WS medium, Chlorella sp. biomass was characterized by a high VS and TOC content as well as C/N ratio, similarly to biomass grown on DW. The effect of different ADEs on organic microalgae components was slight. The average TOC/VS ratio was approximately 5 (p > 0.05). Our study found the impact of ADEs on TN concentration within Chlorella sp. biomass. Higher TN/VS ratio was observed in MS and CM, while lower (p < 0.05) in DW and WS. A higher C/N ratio (p < 0.05) within Chlorella sp. grown on DW and WS than on MS and CM was observed. No significant differences (p > 0.05) were observed as regards TP/VS ratio and pH value in microalgae biomass.
Biogas/Methane Potential of Chlorella sp. Microalgae
The impact of chemical composition of Chlorella sp. biomass grown on ADEs of different characteristics was assessed in mesophilic fermentation batch tests over a period of 20 days.
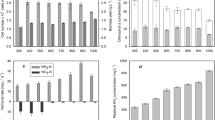
The highest (p < 0.05) cumulative biogas yield (CBY) of 383 mL/g VS was observed in WS variant (Table 4; Fig. 1), where the TOC/VS ratio and C/N ratio within Chlorella sp. biomass were greatest (Table 3). Less CBY was found in DW variant, while the lowest biogas yield (p < 0.05) were obtained during anaerobic digestion of Chlorella sp. cultivated on MS and CM media. Similarly, the cumulative methane yield (CMY) was significantly higher (p < 0.05) in WS and DW variants than that of MA and CM (Table 4). The highest daily biogas production over 40 mL/d was found with MS and DW media (Fig. 2).
Our experimental findings of methane production (183–267 mL CH4/g VS) from Chlorella sp. biomass cultivated on ADEs were similar to the values observed in the previous studies. Methane yield of Chlorella sp. microalgae anaerobic digestion ranged from 123 mL CH4/g VS to 369 mL CH4/g VS [7, 12, 30, 31]. In turn, methane yield of 317.31 mL CH4/g VS was obtained with Chroococcus sp. by Prajapati et al. [15]. Comparing methane yields produced from algae biomass and other organic materials, it could be state that algae biomass is a good source to produce methane. Methane potential with the usage of the other organic materials is as follow: corn straw −216 mL CH4/g VS, rice straw −178 mL CH4/g VS, organic fraction of municipal solid waste −340 mL CH4/g VS, fruit and vegetable wastes −430 mL CH4/g VS, food waste with cattle manure –388 mL CH4/g VS, poultry manure −195 mL CH4/g VS [32], cattle manure −200 mL CH4/g VS, sewage sludge 400 mL CH4/g VS [33].
The CH4 concentration was similar in the biogas produced from the digestion of Chlorella sp. cultivated on DW and WS medium (p > 0.05) (Table 4). Biogas produced in MS and CM variant characterized by a high H2S and NH3 concentration (Table 4). It was indicated that ammonia gas within the digester may have much more inhibitory effect on methanogenic bacteria than the aqueous ionised form of ammonium [23]. This may explain the lowest CMY in MS variant, where the gaseous NH3 concentration was the highest.
The overall biogas production rate (BPR) of 61.28 mL/g VS·d in WS variant and 58.24 mL/g VS·d in DW variant were found (p > 0.05), which was higher (p < 0.05) than in MS and CM variant (Table 4). Lower values have been reported by Prajapati et al. [30]. They achieved the rates of biogas production determined for 30 days of anaerobic digestion of the three species of Chlorella ranged 11.02–17.35 mL/g VS·d.
Our study found a strong correlation between the C/N ratio in microalgae biomass and biogas/methane yield (Fig. 3; Table 5). Similarly, the rate of biogas production, methane and content H2S in biogas were strictly dependent on C/N ratio (Fig. 3; Table 5). The C/N ratio strongly affects the anaerobic digestion thus it should range from 20 to 30 [34]. Low C/N ratio leads to increase ammonia nitrogen liberation and accumulation that may inhibit methanogens [12]. Moreover, ammonia nitrogen increases the pH value in the anaerobic reactor, while the mesophilic digestion is severely inhibited if the pH value rises above pH 8.3 [35]. A long HRT during AD of microalgae biomass can increase nitrogen release [12]. The relationship between the substrate C/N ratio and apparent released ammonia was investigated by Hikada et al. [31]. They found that microalgae biomass consisting mainly of Chlorella sp. released low ammonia, because microalgae contained some non-biodegradable organic residues.
In most cases, microalgae biomass usually contains high amounts of proteins, which is reflected by low C/N of 10 or below. Hidaka et al. [31] reported C/N ratio ranged 2.5–5.3 for Chlorella sp. cultivated on filtrate from dewatering of anaerobically digested sludge. TOC/TNK ratio of Chlorella vulgaris cultivated on synthetic anaerobic digitate was estimated at 6 [12]. In our study, C/N ratio of Chlorella sp. was higher and ranged from 7.2 to 12.9. Zhong et al. [36] suggested an appropriate C/N ratio of 20 for co-digestion microalgae with corn silage. Similarly to our study, Zhao et al. [37] reported an effective methane production from algae biomass having low C/N ratio ranged from 6.8 to 14.8.
Conclusions
Our study found that microalgae were successfully cultivated using various types of anaerobic digestion effluents. Characteristics of the nutrient source used in Chlorella sp. cultivation had a direct effect on C/N ratio in harvested biomass, which was subsequently influenced the biogas yield. The highest C/N ratio was observed when Chlorella sp. was cultured on anaerobically digested effluent of municipal wastewater sludge, while the lowest on anaerobically digested effluents of maize silage and swine slurry. A strong correlation between the C/N ratio value in microalgae biomass and biogas/methane production and rate were observed. It was also demonstrated high biogas production rate of 61.28 mL CH4/g VS from anaerobic digestion of Chlorella sp. cultivated on effluent from anaerobic digestion of municipal wastewater sludge.
References
Xia, A., Murphy, J.D.: Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol. 34, 264–275 (2015)
Cheng, J., Xu, J., Huang, Y., Li, Y., Zhou, J., Cen, K.: Growth optimisation of microalga mutant at high CO2 concentration to purify undiluted anaerobic digestion effluent of swine manure. Bioresour. Technol. 177, 240–246 (2015)
Racharaks, R., Ge, X., Li, Y.: Cultivation of marine microalgae using shale gas flowback water and anaerobic digestion effluent as the cultivation medium. Bioresour. Technol. 191, 146–156 (2015)
Wang, L., Li, Y., Chen, P., Min, M., Chen, Y., Zhu, J., Ruan, R.R.: Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol. 101, 2623–2628 (2010)
Uggetti, E., Sialve, B., Latrille, E., Steyer, J.P.: Anaerobic digestate as substrate for microalgae culture: the role of ammonium concentration on the microalgae productivity. Bioresour. Technol. 152, 437–443 (2014)
Prajapati, S.K., Kaushik, P., Malik, A., Vijay, V.K.: Phycoremediation coupled production of algal biomass, harvesting and anaerobic digestion: possibilities and challenges. Biotechnol. Adv. 31, 1408–1425 (2013)
Wang, M., Sahu, A.K., Rusten, B., Park, C.: Anaerobic co-digestion of microalgae Chlorella sp. and waste activated sludge. Bioresour. Technol. 142, 585–590 (2013)
Patil, V., Tran, K.Q., Giselra˜d, H.R.: Towards sustainable production of biofuels from microalgae. Int. J. Mol. Sci. 9, 1188–1195 (2008)
Li, Y., Horsman, M., Wu, N., Lan, C., Dubois-Calero, N.: Biofuels from microalgae. Biotechnol. Prog. 24, 815–820 (2008)
Sialve, B., Bernet, N., Bernard, O.: Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 27, 409–416 (2009)
Fouilland, E., Vasseur, C., Leboulanger, C., Le Floc’h, E., Carré, C., Marty, B., Steyer, J.P., Sialve, B.: Coupling algal biomass production and anaerobic digestion: production assessment of some native temperate and tropical microalgae. Biomass Bioenergy 70, 564–569 (2014)
Ras, M., Lardon, L., Bruno, S., Bernet, N., Steyer, J.P.: Experimental study on a coupled process of production and anaerobic digestion of Chlorella vulgaris. Bioresour. Technol. 102, 200–206 (2011)
Roberts, K.P., Heaven, S., Banks, C.J.: Comparative testing of energy yields from micro-algal biomass cultures processed via anaerobic digestion. Renew. Energy 87, 744–753 (2016)
Zamalloa, C., Boon, N., Verstraete, W.: Anaerobic digestibility of Scenedesmus obliquus and Phaeodactylum tricornutum under mesophilic and thermophilic conditions. Appl. Energy 92, 733–738 (2012)
Prajapati, S.K., Kumar, P., Malik, A., Vijay, V.K.: Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: a closed loop bioenergy generation process. Bioresour. Technol. 158, 174–180 (2014)
Morales-Amaral, M.M., Gómez-Serrano, C., Acién, F.G., Fernández-Sevilla, J.M., Molina-Grima, E.: Production of microalgae using centrate from anaerobic digestion as the nutrient source. Algal Res. 9, 297–305 (2015)
Yang, L., Tan, X., Li, D., Chu, H., Zhou, X., Zhang, Y., Yu, H.: Nutrients removal and lipids production by Chlorella pyrenoidosa cultivation using anaerobic digested starch wastewater and alcohol wastewater. Bioresour. Technol. 181, 54–61 (2015)
Park, J., Jin, H.F., Lim, B.R., Park, K.Y., Lee, K.: Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Bioresour. Technol. 101, 8649–8657 (2010)
Cai, T., Park, S.Y., Racharaks, R., Li, Y.: Cultivation of Nannochloropsis salina using anaerobic digestion effluent as a nutrient source for biofuel production. Appl. Energy 108, 486–492 (2013)
Ekerlens, M., Ward, A.J., Ball, A.S., Lewis, D.M.: Microalgae digestate effluent as a growth medium for Tetraselmis sp. in the production of biofuels. Bioresour. Technol. 167, 81–86 (2014)
Wang, M., Park, C.: Investigation of anaerobic digestion of Chlorella sp. and Micractinium sp. grown in high-nitrogen wastewater and their co-digestion with waste activated sludge. Biomass Bioenergy 80, 30–37 (2015)
Zhao, Y., Ge, Z., Zhang, H., Bao, J., Sun, S.: Nutrient removal from biogas slurry and biogas upgrading of crude biogas at high CO2 concentrations using marine microalgae. J. Chem. Technol. Biotechnol. 91, 1113–1118 (2015)
Ward, A.J.D., Lewis, M., Green, F.B.: Anaerobic digestion of algae biomass: a review. Algal Res. 5, 204–214 (2014)
Kapdan, I.K., Aslan, S.: Application of the Stover–Kincannon kinetic model to nitrogen removal by Chlorella vulgaris in a continuously operated immobilized photobioreactor system. J. Chem. Technol. Biotechnol. 83, 998–1005 (2008)
Posadas, E., Morales, M.M., Gomez, C., Acién, F.G., Muñoz, R.: Influence of pH and CO2 source on the performance of microalgae-based secondary domestic wastewater treatment in outdoors pilot raceways. Chem. Eng. J. 265, 239–248 (2015)
Park, J.B., Craggs, R.J., Shilton, A.N.: Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 102, 35–42 (2011)
Ogbonna, J.C., Yoshizawa, H., Tanaka, H.: Treatment of high strength organic wastewater by a mixed culture of photosynthetic microorganisms. J. Appl. Phycol. 12, 277–284 (2000)
Muñoz, R., Guieysse, B.: Algal–bacterial processes for the treatment of hazardous contaminants: a review. Water Res. 40, 2799–2815 (2006)
Min, M., Wang, L., Li, Y., Mohr, M.J., Hu, B., Zhou, W., Chen, P., Ruan, R.: Cultivating Chlorella sp. in a pilot-scale photobioreactor using centrate wastewater for microalgae biomass production and wastewater nutrient removal. Appl. Biochem. Biotechnol. 165, 123–137 (2011)
Prajapati, S.K., Malik, A., Vijay, V.K.: Comparative evaluation of biomass production and bioenergy generation potential of Chlorella spp. through anaerobic digestion. Appl. Energy 114, 790–797 (2014)
Hidaka, T., Inoue, K., Suzuki, Y., Tsumori, J.: Growth and anaerobic digestion characteristics of microalgae cultivated using various types of sewage. Bioresour. Technol. 170, 83–89 (2014)
Mao, C., Feng, Y., Wang, X., Ren, G.: Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 45, 540–555 (2015)
Seadi, T.A., Rutz, D., Janssen, R., Drosg, B.: Biomass resources for biogas production. In: Wellinger, A., Murphy, P.J., Baxter, D. (eds.) The Biogas Handbook, Science, Production and Applications, pp. 19–51. Woodhead Publishing, Sawston (2013)
Parkin, G.F., Owen, W.F.: Fundamentals of anaerobic digestion of waste-water sludges. J. Environ. Eng. 112, 867–920 (1986)
Seadi, T.A., Rutz, D., Prassl, H., Kottner, M., Finsterwalder, T., Volk, S., Janssen, R.: Biogas Handbook. University of Southern Denmark Esbjerg, Esbjerg (2008)
Zhong, W., Zhang, Z., Luo, Y., Qiao, W., Xiao, M., Zhang, M.: Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour. Technol. 114, 281–286 (2012)
Zhao, B., Ma, J., Zhao, Q., Laurens, L., Jarvis, E., Chen, S., Frear, C.: Efficient anaerobic digestion of whole microalgae and lipid-extracted microalgae residues for methane energy production. Bioresour. Technol. 161, 423–430 (2014)
Acknowledgments
Research was conducted under Project POIG.01.03.01-26-021/12, entitled: Micro-algae cultivation in closed tubular photobioreactors assisted by both CO2 and other biowastes from a biogas power plant, sponsored by the Programme Innovative Economy within the framework of the European Regional Development Fund, and was also supported by Project No. 18.610.008-300, entitled: Improving the methods of wastewater treatment and sludge processing, from University of Warmia and Mazury in Olsztyn.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dębowski, M., Szwaja, S., Zieliński, M. et al. The Influence of Anaerobic Digestion Effluents (ADEs) Used as the Nutrient Sources for Chlorella sp. Cultivation on Fermentative Biogas Production. Waste Biomass Valor 8, 1153–1161 (2017). https://doi.org/10.1007/s12649-016-9667-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9667-1