Abstract
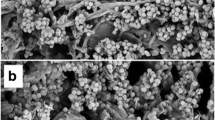
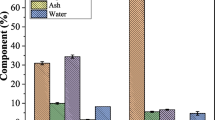
The goal of this study was to screen microorganisms that are potential producers of cellulases, determine optimal conditions for production of these enzymes, and scale up the process, using empty fruit bunches (EFB) as a substrate. Strains of Trichoderma sp., Aspergillus niger, Phanerochaete sp., Ganoderma sp. and Lentinus sp. were evaluated for their ability to produce cellulolytic enzyme complexes under submerged with suspended solids fermentation (SSdF). Among these microorganisms, Phanerochaete sp. (PH-HD), which is already known to be a good producer of lignolytic enzymes, presented the highest capacity for cellulase production. The highest level of cellulase production by Phanerochaete sp. was observed on the 4th day of fermentation at 28 °C and pH 5.7, reaching a value of FPase activity of 364 IU L−1 using 15 g L−1 of EFB as a carbon source, 2 g L−1 of urea as a nitrogen source, 4 g L−1 of KH2PO4, and microcrystalline cellulose Avicel® PH-101 as an inducer (2 g L−1). This study shows the great potential of Phanerochaete sp. for producing cellulolytic enzymes, as well as the feasibility of using EFB under suspended solids fermentation in the production process.





Similar content being viewed by others
References
Uihlein, A., Schebek, L.: Environmental impacts of a lignocellulose feedstock biorefinery system: an assessment. Biomass Bioenergy 33, 793–802 (2009)
Alvarado-Morales, M., Terra, J., Gernaey, K.V., Woodley, J.M., Gani, R.: Biorefining: computer aided tools for sustainable design and analysis of bioethanol production. Chem. Eng. Res. Des. 87, 1171–1183 (2009)
Brehmer, B., Boom, R.M., Sanders, J.: Maximum fossil fuel feedstock replacement potential of petrochemicals via biorefineries. Chem. Eng. Res. Des. 87, 1103–1119 (2009)
Sannigrahi, P., Pu, Y., Ragauskas, A.: Cellulosic biorefineries—unleashing lignin opportunities. Curr. Opin. Environ. Sustain 2, 383–393 (2010)
Sánchez, O.J., Cardona, C.: Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 99, 5270–5295 (2008)
González-García, S., Gasol, C.M., Gabarrell, X., Rieradevall, J., Moreira, M.T., Feijoo, G.: Environmental aspects of ethanol-based fuels from Brassica carinata: a case study of second generation ethanol. Renew. Sustain. Energy Rev. 13, 2613–2620 (2009)
Pérez, J., Muñoz-Dorado, J., de la Rubia, T., Martínez, J.: Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int. Microbiol. 5, 53–63 (2002)
Rutz, D., Janssen, R.: Biofuel Technology Handbook. WIP Renewable Energies, Germany (2008)
Zhang, P.Y.H.: Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 35, 367–375 (2008)
Saini, J.K., Saini, R., Tewari, L.: Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. Biotech 5, 337–353 (2015)
Chiesa, S., Gnansounou, E.: Use of empty fruit bunches from the Oil Palm for bioethanol production: a thorough comparison between dilute acid and dilute alkali pretreatment. Bioresour. Technol. 159, 355–364 (2014)
Jung, Y.H., Kim, S., Yang, T.H., Lee, H.J., Seung, D., Park, Y.C.: Aqueous ammonia pretreatment, saccharification and fermentation evaluation of oil palm fronds for ethanol production. Bioprocess Biosyst. Eng. 35, 1467–1475 (2012)
Kang Q., Appels L., Tan T., Dewil R.: Bioethanol from lignocellulosic biomass: current findings determine research priorities. Sci. World J. 2014, 1–13 (2014)
Juturu, V., Wu, J.C.: Microbial cellulases: engineering, production and applications. Renew. Sustain. Energy Rev. 33, 188–203 (2014)
Sadhu, S., Maiti, T.K.: Cellulase production by bacteria: a review. Br. Microbiol. Res. J. 3(3), 235–258 (2013)
Verardi, A., Bari, I., Ricca, E., Calabrò, V.: Hydrolysis of lignocellulosic biomass: current status of processes and technologies and future perspectives. In: Lima, A.P., Natalense, P.P. (eds.) Bioethanol, pp. 95–122. InTech-Open Access Publisher, Rijeka (2012). doi:10.5772/23987
Sternberg, D., Mandels, G.R.: Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J. Bacteriol. 139, 761–769 (1979)
Chen, C., Wayman, M.: Novel inducers derived from starch for cellulase production by Trichoderma reesei. Process Biochem. 27, 327–334 (1992)
Kuhad R.C., Gupta R., Singh A.: Microbial cellulases and their industrial applications. Enzyme Res. 2011, (2011). doi:10.4061/2011/280696
Jørgensen, H., Mørkeberg, A., Krogh, K.B.R., Olsson, L.: Production of cellulases and hemicellulases by three Penicillium species: effect of substrate and evaluation of cellulase adsorption by capillary electrophoresis. Enzyme Microb. Technol. 36, 42–48 (2005)
Krogh, K.B.R., Mørkeberg, A., Jørgensen, H., Frisvad, J.C., Olsson, L.: Screening genus Penicillium for producers of cellulolytic and xylanolytic enzymes. Appl. Biochem. Biotechnol. 113–116, 389–401 (2004)
Zhi, Z., Wang, H.: White-rot fungal pretreatment of wheat straw with Phanerochaetechrysosporium for biohydrogen production: simultaneous saccharification and fermentation. Bioprocess Biosyst. Eng. 37, 1447–1458 (2014)
Uzcategui, E., Raices, M., Montesino, R., Johansson, G., Pettersson, G., Eriksson, K.E.: Pilot-scale production and purification of the cellulolytic enzyme system from the white-rot fungus Phanerochaetechrysosporium. Biotechnol. Appl. Biochem. 13, 323–334 (1991)
Kim, D.M., Cho, E.J., Kim, J.W., Lee, Y.W., Chung, H.J.: Production of cellulases by Penicillium sp. in a solid-state fermentation of oil palm empty fruit bunch. Afr. J. Biotechnol. 13, 145–155 (2014)
Baharuddin, A.S., Nafis, M., Razak, A., Hock, L.S., Ahmad, M.N.: Isolation and characterization of thermophilic cellulase-producing bacteria from empty fruit bunches-palm oil mill effluent compost. Am. J. Appl. Sci. 7, 56–62 (2010)
Alam, M.Z., Muhammad, N., Mahmat, M.E.: Production of cellulase from oil palm biomass substrate by solid state bioconversion. Am. J. Appl. Sci. 2, 569–572 (2005)
Mrudula, S., Murugammal, R.: Production of cellulase by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Braz. J. Microbiol. 42, 1119–1127 (2011)
Li, X., Yang, H., Roy, B., Park, E.Y., Jiang, L., Wang, D., Miao, Y.: Enhanced cellulase production of the Trichoderma viride mutated by microwave and ultraviolet. Microbiol. Res. 165, 190–198 (2010)
Salmon, D.N.X., Spier, M.R., Soccol, C.R., Vandenberghe, L.P.D.S., WeingartnerMontibeller, V., Bier, M.C.J., et al.: Analysis of inducers of xylanase and cellulase activities production by Ganodermaapplanatum LPB MR-56. Fungal Biol. 118, 655–662 (2014)
Mary, M., James, W.: The Production of Cellulases. Advances in chemistry, Massachusetts (2009)
Ghose, T.K.: Measurement of cellulase activities. Int. Union Pure Appl. Chem. 59, 257–268 (1987)
Rodrigues-Gomez, D., Hobley, T.J.: Is an organic nitrogen source needed for cellulase production by Trichoderma reesei Rut-C30? World J. Microbiol. Biotechnol. 29, 2157–2165 (2013)
Ashter, M., Capdevilla, C., Corrieu, G.: Control on lignin peroxidase production by Phanerochaete chrysosporium INA-12 by temperature shifting. Appl. Environ. Microbiol. 54, 3194–3196 (1988)
Hatakka, A., Uusi-Rauva, A.K.: Degradation of 14C-labelled poplar wood lignin by selected white-rot fungi. Eur. J. Appl. Microbiol. Biotechnol. 17, 235–242 (1983)
Umikalson, M.S., Ariff, A.B., Zulkifli, H.S., Tong, C.C., Hassan, M.A., Karim, M.A.: The treatment of oil palm empty fruit bunch fibre for subsequent use as substrate for cellulase production by chaetomium globosum kunze. Bioresour. Technol. 62, 1–9 (1997)
Narasimha, G., Sridevi, A., Buddolla, V., Subhosh, C.M., Rajasekhar, R.B.: Nutrient effects on production of cellulolytic enzymes by Aspergillus niger. Afr. J. Biotechnol. 5, 472–476 (2006)
Türker, M., Mavituna, F.: Production of cellulase by freely suspended and immobilized cells of Trichodermareesei. Enzyme Microb. Technol. 9, 739–743 (1987)
Ratha, J., Adthalungrong, C.: Selection of nutrient parameters for endoglucanase production from rice bran by Penicilliumsp. using Plackett-Burman design. KKU Res. J. 17, 965–971 (2012)
El-sersy, N.A., Abd-elnaby, H., Abou-elela, G.M., Ibrahim, H.A.H., El-toukhy, N.M.K.: Optimization, economization and characterization of cellulase produced by marine Streptomyces ruber. Afr. J. Biotechnol. 9, 6355–6364 (2010)
Ali, S.B.R., Muthuvelayudham, R., Viruthagiri, T.: Enhanced production of cellulase from tapioca stem using response surface methodology. Innov. Rom. Food Biotechnol. 12, 40–51 (2013)
Khalil, A.I.: Production and characterization of cellulolytic and xylanolytic enzymes from the ligninolytic white-rot fungus Phanerochaetechrysosporium grown on sugarcane bagasse. World J. Microbiol. Biotechnol. 18, 753–759 (2002)
Khan, M.H., Ali, S., Fakhru’l-Razi, A., Alam, Z.: Use of fungi for the bioconversion of rice straw into cellulase enzyme. J. Environ. Sci. Heal. Part B 42, 381–386 (2007)
Matkar, K., Chapla, D., Divecha, J., Nighojkar, A., Madamwar, D.: Production of cellulase by a newly isolated strain of Aspergillus sydowii and its optimization under submerged fermentation. Int. Biodeterior. Biodegrad. 78, 24–33 (2013)
Acknowledgments
This work was supported by the Co-Operative Project between Vale S.A. and UFPR intitled BIOPAL—Integrated Biorefineries to Sustainable Processing of Palm—Biological Route Subproject C1—Residues from palm as substrate to production of the Cellulolytc Enzimatic Complex.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maceno, M.A.C., de Souza Vandenberghe, L.P., Woiciechowski, A.L. et al. Production of Cellulases by Phanerochaete sp. Using Empty Fruit Bunches of Palm (EFB) as Substrate: Optimization and Scale-Up of Process in Bubble Column and Stirred Tank Bioreactors (STR). Waste Biomass Valor 7, 1327–1337 (2016). https://doi.org/10.1007/s12649-016-9503-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9503-7