Abstract
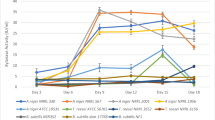
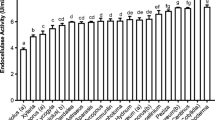
With the aim to utilize deoiled rice bran, an agro-industrial waste, as a feedstock for the co-production of multiple carbohydrases, a fungal strain was isolated which could utilize DORB to co-produce a consortium of cellulases, hemicellulases, pectinase and amylases and was named as Aspergillus niger P-19 after molecular identification. Further, optimization for the co-production of all the enzymes was carried out by one factor at a time approach. Time profile studies of the production of enzymes revealed that 5th day of incubation was best suited for the extraction of enzymes. An initial solid to moisture ratio of 1:1.5 and an inoculum size of 5 × 107 spores gds−1 were found to be optimum for maximum productivities. Enzyme yields were significantly improved with the exogenous supplementation of carbon source, nitrogen source, surfactants and lignocellulosic inducers. This is the first report of its kind where DORB has been utilized for the co-production and co-optimization of eight different enzymes which can have a potential application in biofuel industry as the enzyme preparation could effectively hydrolyze steam pre-treated DORB releasing a total reducing sugars of 356.17 ± 9.58 mg gds−1.

Similar content being viewed by others
References
Nigam, P.S., Singh, A.: Production of liquid biofuels from renewable resources. Prog. Energ. Combust. 37, 52–68 (2011)
Biernat, K., Malinowski, A., Gnat, M.: The possibility of future biofuels production using waste carbon dioxide and solar energy. In: Fang, Z. (ed.) Biofuels-Economy, Environment and Sustainability (2013). doi:10.5772/53831
Maitan-Alfenas, G.P., Visser, E.M., Guimares, V.M.: Enzymatic hydrolysis of lignocellulosic biomass: converting food waste in valuable products. Curr. Opin. Food Sci. 1, 44–49 (2015)
Poli, A., Anzelmo, G., Fiorentino, G., Nicolaus, B. et al.: Polysaccharides from wastes of vegetable industrial processing: new opportunities for their eco-friendly re-use. In: Elnashar, M. (ed.) Biotechnology of Biopolymers (2011). doi:10.5772/16387
Soni, S.K., Batra, N., Bansal, N., Soni, R.: Bioconversion of sugarcane baggase into second generation bioethanol after enzymatic hydrolysis with in-house produced cellulases from Aspergillus sp. S4B2F. BioRes. 5, 741–758 (2010)
Yang, S.Q., Yan, Q.J., Jiang, Z.Q., Li, L.T., et al.: High-level of xylanase production by the thermophilic Paecilomyces themophila J18 on wheat straw in solid-state fermentation. Bioresour. Technol. 97, 1794–1800 (2006)
Kang, S.W., Park, Y.S., Lee, J.S., Hong, S.I., et al.: Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour. Technol. 91, 153–156 (2004)
Vyas, A., Vyas, D.: Production of fungal cellulases by solid state bioprocessing of groundnut shell wastes. J. Sci. Ind. Res. 64, 767–770 (2005)
Kheng, P.P., Omar, I.C.: Xylanase production by a local fungal isolate, Aspergillus niger USM AI 1 via solid state fermentation using palm kernel cake (PKC) as substrate. Songklanakarin J. Sci. Technol. 27, 325–336 (2005)
Pothiraj, C., Balaji, P., Eyini, M.: Enhanced production of cellulases by various fungal cultures in solid state fermentation of cassava waste. Afr. J. Biotechnol. 5, 1882–1885 (2006)
Gao, J., Weng, H., Zhu, D., Yuan, M.: Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Bioresour. Technol. 99, 7623–7629 (2008)
Samuel, S., Muthukkaruppan, S.M., Gayathri, S.N., Kumar, P.K.: Cellulase production by Bacillus spp and Aspergillus niger using coir waste and saw dust and partial purification. Int. J. Curr. Res. 2, 31–34 (2010)
Brijwani, K., Oberoi, H.S., Vadlani, P.V.: Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochem. 45, 120–128 (2010)
Juwaied, A.A., Adnan, S., Al-Amiery, A.A.H.H.: Production of cellulase by different co- culture of Aspergillus niger and Tricoderma viride from waste paper. J. Yeast Fungal Res. 1, 108–111 (2010)
Janveja, C., Rana, S.S., Soni, S.K.: Environmentally acceptable management of kitchen waste residues by using them as substrates for the co-production of a cocktail of fungal carbohydrases. Int. J. Chem. Environ. Eng. Sys. 4, 20–29 (2013)
Rana, S.S., Janveja, C., Soni, S.K.: A β-mannanase from Fusarium oxysporum SS-25 via solid state fermentation on brewer’s spent grain: medium optimization by statistical tools, kinetic characterization and its applications. Int. J. Biol. Vet. Agr. Eng. 9, 115–125 (2015)
Department of Agriculture and Cooperation, India: Commodity Profile for Rice-March 2015. http://agricoop.nic.in/imagedefault/trade/Ricenew.pdf (2015). Accessed 24 July 2015
Kahlon, T.S.: Rice Bran: production, composition, functionality and food applications, physiological benefits. In: Cho, S.S., Samuel, P. (eds.) Food Applications and Health Benefits, pp. 305–321. CRC Press, Florida (2009)
Grover, A., Maninder, A., Sarao, L.K.: Production of fungal amylase and cellulase enzyme via solid state fermentation using Aspergillus oryzae and Trichoderma reesei. Int. J. Adv. Res. Technol. 2, 108–124 (2013)
Deswal, D., Khasa, Y.P., Kuhad, R.C.: Optimization of cellulase production by a brown rot fungus Famitopsis sp. RCK2010 under solid state fermentation. Bioresour. Technol. 102, 6065–6072 (2011)
Singhania, R.R., Sukumaran, R.K., Pillai, A., Prema, P., et al.: Solid-state fermentation of lignocellulosic substrates for cellulase production by Trichoderma reesei NRRL 11460. Indian J. Biotechnol. 5, 332–336 (2006)
Lee, C.K., Darah, I., Ibrahim, C.O.: Production and optimization of cellulase enzyme using Aspergillus niger USM AI 1 and comparison with Trichoderma reesei via solid state fermentation system. Biotechnol. Res. Int. (2011). doi:10.4061/2011/658493
Puri, S., Arora, M., Sarao, L.: Production and optimization of amylase and glucoamylase using Aspergillus oryzae under solid state fermentation. Int. J. Res. Pure Appl. Microbiol. 3, 83–88 (2013)
Rana, S.S., Janveja, C., Soni, S.K.: Brewer’s spent grain as a valuable substrate for low cost production of fungal cellulases by statistical modelling in solid state fermentation and generation of cellulosic ethanol. Int. J. Food. Ferment. Technol. 3, 41–55 (2013)
Janveja, C., Rana, S.S., Soni, S.K.: Optimization of valorization of biodegradable kitchen waste biomass for production of fungal cellulase system by statistical modelling. Waste Biomass Valorization 5, 807–821 (2014)
Tamura, K., Dudley, J., Nei, M., Kumar, S.: MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 (2007)
Mandels, M., Andreotti, R.E., Roche, C.: Measurements of saccharifying cellulases. Biotechnol. Biophys. Symp. 6, 21–23 (1976)
Bailey, M.J., Biley, P., Poutanen, K.: Inter laboratory testing of methods for assay of xylanase activity. J. Biotechnol. 23, 257–270 (1992)
Stalbrand, H., Siika-aho, M., Viikari, L.: Purification and characterization of two β-mannanases from Trichoderma reesei. J. Biotechnol. 29, 229–242 (1993)
Minjares-Carranco, A., Trejo-Aguilar, B.A., Guillermo, A., Viniegra-Gonzalez, G.: Physiological comparision between pectinase producing mutants of Aspergillus niger adopted either to solid state fermentation or submerged fermentation. Enzyme Microb. Technol. 21, 25–31 (1997)
Fuwa, H.: A new method for micro determination of amylase activity by the use of amylose as substrate. J. Biochem. 41, 583–603 (1954)
Cori, G.T.: Amylo-1,6-glucosidase. Methods Enzymol. 1, 211–214 (1955)
Dubois, M., Gilles, K.A., Hamilton, K., Rebers, P.A., Smith, F.: Calorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
Miller, G.L.: Use of DNS reagent for determination of reducing sugars. Anal. Chem. 31, 426–428 (1959)
Morin, L.G., Prox, J.: Single glucose oxidase-peroxidase reagent for two-minute determination of serum glucose. Clinical Chem. 19, 959–962 (1973)
Pirota, R.D.P.B., Delabona, P.S., Farinas, C.S.: Enzymatic hydrolysis of sugarcane bagasse using enzyme extract and whole solid-state fermentation medium of two newly isolated strains of Aspergillus Oryzae. Chem. Eng. Trans. 38, 259–264 (2014)
Camassola, M., Dillon, A.J.P.: Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid-state fermentation. J. Appl. Microbiol. 103, 2196–2204 (2007)
Rehman, S., Aslam, H., Ahmad, A., Khan, S.A., et al.: Production of plant cell wall degrading enzymes by monoculture and co-culture of Aspergillus niger and Aspergillus terreus under SSF of banana peels. Braz. J. Microbiol. 45, 1485–1492 (2014)
Ang, S.K., Shaza, E.M., Adibah, Y., Suraini, A.A., et al.: Production of cellulases and xylanase by Aspergillus fumigates SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem. 48, 1293–1302 (2013)
Dhillon, G.S., Kaur, S., Brar, S.K., Verma, M.: Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulases bioproduction through solid-state fermentation. Ind. Crop Prod. 38, 6–13 (2012)
Kumar, S., Sharma, H.K., Sarkar, B.C.: Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in submerged (SmF) and solid state fermentation (SSF). Food Sci. Biotechnol. 20, 1289–1298 (2011)
Bansal, N., Tewari, R., Gupta, J.K., Soni, R., et al.: A novel strain of Aspergillus niger producing a cocktail of hydrolytic depolymerising enzymes for the production of second generation biofuels. BioRes. 6, 552–569 (2011)
Bansal, N., Tewari, R., Soni, R., Soni, S.K.: Production of cellulases from Aspergillus niger NS-2 in solid state fermentation on agricultural and kitchen waste residues. Waste Manage. 7, 1341–1346 (2012)
Maurya, D.P., Singh, D., Pratap, D., Maurya, J.P.: Optimization of solid state fermentation conditions for the production of cellulase by Trichoderma reesei. J. Environ. Biol. 33, 5–8 (2012)
Sohail, M., Siddiqi, R., Ahmad, A., Khan, S.A.: Cellulase production from Aspergillus niger MS82: effect of temperature and pH. New Biotechnol. 25, 437–441 (2009)
Baysol, Z., Uyar, F., Aytekin, C.: Solid state fermentation for production of alpha amylase by a thermotolerant Bacillus subtilis from hot spring water. Process Biochem. 38, 1665–1668 (2003)
Ahmed, S., Bashir, A., Saleem, H., Saadia, M., et al.: Production and purification of cellolose-degrading enzymesfrom a filamentous fungus Trichoderma herzanium. Pak. J. Bot. 41, 1411–1419 (2009)
Soni, S.K., Soni, R.: Regulation of cellulase synthesis in Chaetomium erraticum. BioRes. 5, 81–98 (2010)
Sohail, M., Ahmad, A., Khan, S.A.: Production of cellulases from Alternaria sp. MS28 and their partial characterization. Pak. J. Bot. 43, 3001–3006 (2011)
Balakrishnan, K., Kumar, R., Devi, R.A., Jayasri, S., et al.: Utilization of fortified rice husk for the fermentative production of xylanase by Trichoderma sp. Int J. Curr. Microbiol. Appl. Sci. 2, 174–187 (2013)
Chantorn, S.T., Buengsrisawat, K., Pokaseam, A., Sombat, T., et al.: Optimization of extracellular mannanase production from Penicillium oxalicum KUB-SN2-1 and application for hydrolysis property. Songklanakarin J. Sci. Technol. 35, 17–22 (2013)
Erdal, S., Taskin, M.: Production of α-amylase by Penicillium expansum MT-1in solid state fermentation using waste Loquat (Eriobotrya japonica Lindley) kernels as substrate. Rom. Biotechnol. Lett. 15, 5342–5350 (2010)
Sivaramakrishnan, S., Gangadharan, D., Nampoothiri, K.M., Soccol, C.R., et al.: Alpha amylase production by Aspergillus oryzae employing solid state fermentation. J. Sci. Ind. Res. 66, 621–626 (2007)
Padmavathi, T., Agarwal, P., Nandy, V.: Exploring marine fungal strains for cellulase production. Ann. Biol. Res. 3, 3602–3613 (2012)
Akinyele, J.B., Fabunmi, A.O., Olaniyi, O.O.: Effect of variations in growth parameters in cellulase activity of Trichoderma viride NSPR006 cultured on different wood-dusts. Malays. J. Microbiol. 9, 193–200 (2013)
Azzaz, H.H., Murad, H., Kholif, A.M., Hanfy, M.A., et al.: Optimization of culture conditions affecting fungal cellulase production. Res. J. Microbiol. 7, 23–31 (2012)
Kachlishvili, E., Penninckx, M.J., Tsiklauri, N., Elisashvili, V.: Effect of nitrogen source on lignocellulolytic enzyme production by white-rot basidiomycetes under solid-state cultivation. World J. Microbiol. Biotechnol. 22, 391–397 (2006)
Bansal, N., Janveja, C., Tewari, R., Soni, R., et al.: Highly thermostable and ph-stable cellulases from Aspergillus niger NS-2: properties and application for cellulose hydrolysis. Appl. Biochem. Biotechnol. 172, 141–156 (2014)
Goyal, M., Kalra, K.L., Sareen, V.K., Soni, G.: Xylanase production with xylan rich lignocellulosic wastes by a local soil isolate of Trichoderma viride. Braz. J. Microbiol. 39, 535–541 (2008)
Rana, S.S., Janveja, C., Soni, S.K.: Statistical modeling for enhanced xylanase production by Fusarium oxysporum SS-25 via solid state fermentation of Brewer’s spent grain. J. Technol. Innov. Renew. Energy 2, 173–185 (2013)
Bhardwaj, S., Vedamurthy, A.B., Bhattacharya, S., Das, A.: Effect of inorganic salts and surfactants on the production of α-amylase by a Mangrove isolate of Aspergillus flavus using solid-state fermentation. J. Chem. Biol. Phys. Sci. 2, 1390–1397 (2012)
Pardo, A.G.: Effect of surfactants on cellulase production by Nectria catalinensis. Curr. Microbiol. 33, 275–278 (1996)
Evans, E.C., Abdullahi, A.: Effect of surfactant inclusions on the yield and characteristics of protease from Bacillus subtilis. Proc. Rom. Acad., Series B 2, 108–112 (2012)
Uyar, F., Porsuk, I., Kizil, G., Yilmaz, E.I.: Optimal conditions for production of extracellular protease from newly isolated Bacillus cereus strain CA15. EurAsian J. BioSci. 5, 1–9 (2011)
Ikram-ul-haq, Shamim, N., Ashraf, H., Ali, S. et al.: Effect of surfactants on the biosynthesis of alpha amylase by Bacillus subtilis GCBM-25. Pak. J. Bot. 37, 373–379 (2005)
Usha, K.Y., Praveen, K., Reddy, B.R.: Enhanced production of ligninolytic enzymes by a mushroom Stereum ostrea. Biotechnol. Res. Int. (2014). doi:10.1155/2014/815495
Al-Asheh, S., Duvnjak, Z.: The effect of surfactants on the phytase production and the reduction of the phytic acid content in canola meal by Aspergillus carbonarius during a solid state fermentation process. Biotechnol. Lett. 16, 183–188 (1994)
Shankar, T., Isaiarasu, L.: Cellulase production by Bacillus pumilus EWBCM1 under varying cultural conditions. Middle-East J. Sci. Res. 8, 40–45 (2011)
Shahriarinour, M., Wahab, M.N.A., Mohamad, R., Mustafa, S., et al.: Effect of medium composition and cultural condition on cellulase production by Aspergillus terreus. Afr. J. Biotechnol. 10, 7459–7467 (2011)
Mehboob, N., Asad, M.J., Asgher, M., Gulfraz, M., et al.: Exploring thermophillic cellulolytic enzyme production potential of Aspergillus fumigates by the solid-state fermentation of wheat straw. Appl. Biochem. Biotechnol. 172, 3646–3655 (2014)
Reddy, G.P.K., Narasimha, G., Kumar, K.D., Ramanjaneyulu, G., et al.: Cellulase production by Aspergillus niger on different natural lignocellulosic substrates. Int. J. Curr. Microbiol. Appl. Sci. 4, 835–845 (2015)
Acknowledgments
Authors are highly thankful for the financial assistance provided by Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India and Department of Science and Technology (DST). Junior Research Fellowship awarded under DST-INSPIRE scheme to Ms. Priya is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chugh, P., Soni, R. & Soni, S.K. Deoiled Rice Bran: A Substrate for Co-Production of a Consortium of Hydrolytic Enzymes by Aspergillus niger P-19. Waste Biomass Valor 7, 513–525 (2016). https://doi.org/10.1007/s12649-015-9477-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-015-9477-x