Abstract
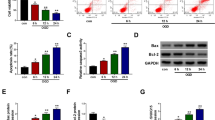
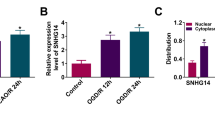
Background Emerging studies illustrate that long non-coding RNA TUG1 (TUG1) participates in neuron death after ischemia. However, the role of TUG1 in cerebral ischemia/reperfusion (CI/R) injury through cerebrovascular pathology was undetermined yet. Methods Expression of TUG1, miRNA-410-3p (miR-410), and forkhead box O3 (FOXO3) was detected by RT-qPCR and western blot. Neural function, apoptosis, and inflammatory damage were assessed by triphenyltetrazolium chloride straining, modified neurological severity score, fluorescence-activated cell sorting method, and western blot. The relationship among TUG1, miR-410, and FOXO3 was identified by dual-luciferase reporter assay, RNA pull-down, and RNA immunoprecipitation. Results TUG1 was upregulated in middle cerebral artery occlusion/reperfusion (MCAO/R) mice and oxygen–glucose deprivation/reoxygenation (OGD/R)-induced mouse brain microvascular endothelial cells (BMECs) in a certain of time-dependent manner. Blockage of TUG1 decreased infarct volume and increased neurological score in MCAO/R mice, accompanied with elevated Bcl-2 expression and declined expression of IL-1β, IL-6, TNF-α, Bax, and cleaved caspase 3. Abovementioned proteins were similarly expressed in OGD/R-induced BMECs with TUG1 knockdown, paralleled with diminished apoptosis rate. Either, miR-410 overexpression and FOXO3 interference could suppress OGD/R-induced inflammatory and apoptotic responses. Of note, TUG1 and FOXO3 are competing endogenous RNAs (ceRNAs) for miR-410 via target binding. Depleting miR-410 counteracted the role of TUG1 exhaustion, and reinforcing FOXO3 abated the effect of miR-410 overexpression. Conclusion Exhausting TUG1 could alleviate CI/R-induced inflammatory injury and apoptosis in brain tissues and BMECs via targeting miR-410/FOXO3 axis, suggesting an innovative perspective from cerebrovascular endothelial cells in the pathogenesis and treatment of CI/R.








Similar content being viewed by others
References
Akella A, Bhattarai S, Dharap A (2019) Long noncoding RNAs in the pathophysiology of ischemic stroke. Neuromolecular Med 21(4):474–483. https://doi.org/10.1007/s12017-019-08542-w
Bao MH, Szeto V, Yang BB, Zhu SZ, Sun HS, Feng ZP (2018) Long non-coding RNAs in ischemic stroke. Cell Death Dis 9(3):281. https://doi.org/10.1038/s41419-018-0282-x
Basile DP (2007) The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72(2):151–156. https://doi.org/10.1038/sj.ki.5002312
Chen S, Wang M, Yang H, Mao L, He Q, Jin H, Ye ZM, Luo XY, Xia YP, Hu B (2017) LncRNA TUG1 sponges microRNA-9 to promote neurons apoptosis by up-regulated Bcl2l11 under ischemia. Biochem Biophys Res Commun 485(1):167–173. https://doi.org/10.1016/j.bbrc.2017.02.043
Du J, Li W, Wang B (2021) Long non-coding RNA TUG1 aggravates cerebral ischemia and reperfusion injury by sponging miR-493-3p/miR-410-3p. Open Med (wars) 16(1):919–930. https://doi.org/10.1515/med-2021-0253
El Amki M, Wegener S (2017) Improving cerebral blood flow after arterial recanalization: a novel therapeutic strategy in stroke. Int J Mol Sci 18(12). https://doi.org/10.3390/ijms18122669
Ghafouri-Fard S, Shoorei H, Taheri M (2020) Non-coding RNAs participate in the ischemia-reperfusion injury. Biomed Pharmacother 129:110419. https://doi.org/10.1016/j.biopha.2020.110419
Guo D, Ma J, Li T, Yan L (2018) Up-regulation of miR-122 protects against neuronal cell death in ischemic stroke through the heat shock protein 70-dependent NF-kappaB pathway by targeting FOXO3. Exp Cell Res 369(1):34–42. https://doi.org/10.1016/j.yexcr.2018.04.027
Guo D, Ma J, Yan L, Li T, Li Z, Han X, Shui S (2017) Down-regulation of Lncrna MALAT1 attenuates neuronal cell death through suppressing Beclin1-dependent autophagy by regulating Mir-30a in cerebral ischemic stroke. Cell Physiol Biochem 43(1):182–194. https://doi.org/10.1159/000480337
Hu MY, Du XB, Hu HB, Shi Y, Chen G, Wang YY (2018) MiR-410 inhibition induces HUVECs proliferation and represses ox-LDL-triggered apoptosis through activating STAT3. Biomed Pharmacother 101:585–590. https://doi.org/10.1016/j.biopha.2018.02.111
Li L, Li L, Zhang YZ, Yang HY, Wang YY (2020) Long non-coding RNA FTX alleviates hypoxia/reoxygenation-induced cardiomyocyte injury via miR-410–3p/Fmr1 axis. Eur Rev Med Pharmacol Sci 24(1):396–408. https://doi.org/10.26355/eurrev_202001_19938
Li L, Zhang Q, Wang Y, Yin S, Chi S, Han F, Wang W (2021) Knockdown of lncRNA TUG1 attenuates cerebral ischemia/reperfusion injury through regulating miR-142-3p. BioFactors. https://doi.org/10.1002/biof.1765
Li Z, Li J, Tang N (2017) Long noncoding RNA Malat1 is a potent autophagy inducer protecting brain microvascular endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury by sponging miR-26b and upregulating ULK2 expression. Neuroscience 354:1–10. https://doi.org/10.1016/j.neuroscience.2017.04.017
Liu J, Zhang KS, Hu B, Li SG, Li Q, Luo YP, Wang Y, Deng ZF (2018a) Systematic analysis of RNA regulatory network in rat brain after ischemic stroke. Biomed Res Int 2018:8354350. https://doi.org/10.1155/2018/8354350
Liu NN, Dong ZL, Han LL (2018b) MicroRNA-410 inhibition of the TIMP2-dependent MAPK pathway confers neuroprotection against oxidative stress-induced apoptosis after ischemic stroke in mice. Brain Res Bull 143:45–57. https://doi.org/10.1016/j.brainresbull.2018.09.009
Luo Y, Tang H, Li H, Zhao R, Huang Q, Liu J (2018) Recent advances in the development of neuroprotective agents and therapeutic targets in the treatment of cerebral ischemia. Eur J Med Chem 162:132–146. https://doi.org/10.1016/j.ejmech.2018.11.014
Maiese K, Hou J, Chong ZZ, Shang YC (2009) A fork in the path: developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev 2(3):119–129. https://doi.org/10.4161/oxim.2.3.8916
Nan S, Wang Y, Xu C, Wang H (2021) Interfering microRNA-410 attenuates atherosclerosis via the HDAC1/KLF5/IKBalpha/NF-kappaB axis. Mol Ther Nucleic Acids 24:646–657. https://doi.org/10.1016/j.omtn.2021.03.009
Nour M, Scalzo F, Liebeskind DS (2013) Ischemia-Reperfusion Injury in Stroke Interv Neurol 1(3–4):185–199. https://doi.org/10.1159/000353125
Ong SB, Katwadi K, Kwek XY, Ismail NI, Chinda K, Ong SG, Hausenloy DJ (2018) Non-coding RNAs as therapeutic targets for preventing myocardial ischemia-reperfusion injury. Expert Opin Ther Targets 22(3):247–261. https://doi.org/10.1080/14728222.2018.1439015
Shan W, Chen W, Zhao X, Pei A, Chen M, Yu Y, Zheng Y, Zhu S (2020) Long noncoding RNA TUG1 contributes to cerebral ischaemia/reperfusion injury by sponging mir-145 to up-regulate AQP4 expression. J Cell Mol Med 24(1):250–259. https://doi.org/10.1111/jcmm.14712
She DT, Wong LJ, Baik SH, Arumugam TV (2018) SIRT2 inhibition confers neuroprotection by downregulation of FOXO3a and MAPK signaling pathways in ischemic stroke. Mol Neurobiol 55(12):9188–9203. https://doi.org/10.1007/s12035-018-1058-0
Su SH, Wu CH, Chiu YL, Chang SJ, Lo HH, Liao KH, Tsai CF, Tsai TN, Lin CH, Cheng SM, Cheng CC, Wang HW (2017) Dysregulation of vascular endothelial growth factor receptor-2 by multiple miRNAs in endothelial colony-forming cells of coronary artery disease. J Vasc Res 54(1):22–32. https://doi.org/10.1159/000449202
Wang H, Liao S, Li H, Chen Y, Yu J (2019a) Long non-coding RNA TUG1 sponges Mir-145a-5p to regulate microglial polarization after oxygen-glucose deprivation. Front Mol Neurosci 12:215. https://doi.org/10.3389/fnmol.2019.00215
Wang Q, Liu X, Zhu R (2019b) Long noncoding RNAs as diagnostic and therapeutic targets for ischemic stroke. Curr Pharm Des 25(10):1115–1121. https://doi.org/10.2174/1381612825666190328112844
Wang XX, Zhang B, Xia R, Jia QY (2020) Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci 24(18):9601–9614. https://doi.org/10.26355/eurrev_202009_23048
Wei YS, Yang J, He YL, Shi X, Zeng ZN (2019) A functional polymorphism in the promoter of TUG1 is associated with an increased risk of ischaemic stroke. J Cell Mol Med 23(9):6173–6181. https://doi.org/10.1111/jcmm.14499
Xiong ZJ, Zhang Q, Wang DX, Hu L (2018) Overexpression of TUG1 promotes neuronal death after cerebral infarction by regulating microRNA-9. Eur Rev Med Pharmacol Sci 22(21):7393–7400. https://doi.org/10.26355/eurrev_201811_16278
Xu W, Gao L, Zheng J, Li T, Shao A, Reis C, Chen S, Zhang J (2018) The roles of microRNAs in stroke: possible therapeutic targets. Cell Transplant 27(12):1778–1788. https://doi.org/10.1177/0963689718773361
Yang D, Li Z, Gao G, Li X, Liao Z, Wang Y, Li W, Zhang Y, Liu W (2021) Combined analysis of surface protein profile and microRNA expression profile of exosomes derived from brain microvascular endothelial cells in early cerebral ischemia. ACS Omega 6(34):22410–22421. https://doi.org/10.1021/acsomega.1c03248
Yang F, Li T, Dong Z, Mi R (2018) MicroRNA-410 is involved in mitophagy after cardiac ischemia/reperfusion injury by targeting high-mobility group box 1 protein. J Cell Biochem 119(2):2427–2439. https://doi.org/10.1002/jcb.26405
Yin J, Han P, Tang Z, Liu Q, Shi J (2015) Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J Cereb Blood Flow Metab 35(11):1783–1789. https://doi.org/10.1038/jcbfm.2015.123
Yin M, Chen WP, Yin XP, Tu JL, Hu N, Li ZY (2021) LncRNA TUG1 demethylated by TET2 promotes NLRP3 expression, contributes to cerebral ischemia/reperfusion inflammatory injury. ASN Neuro 13:17590914211003247. https://doi.org/10.1177/17590914211003247
Young TL, Matsuda T, Cepko CL (2005) The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol 15(6):501–512. https://doi.org/10.1016/j.cub.2005.02.027
Yu S, Yu M, He X, Wen L, Bu Z, Feng J (2019) KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7 pathway in cerebral ischemic stroke. Aging Cell 18(3):e12940. https://doi.org/10.1111/acel.12940
Zhang C, Huang J, An W (2017a) Hepatic stimulator substance resists hepatic ischemia/reperfusion injury by regulating Drp1 translocation and activation. Hepatology 66(6):1989–2001. https://doi.org/10.1002/hep.29326
Zhang T, Wang H, Li Q, Fu J, Huang J, Zhao Y (2018) MALAT1 activates the P53 signaling pathway by regulating MDM2 to promote ischemic stroke. Cell Physiol Biochem 50(6):2216–2228. https://doi.org/10.1159/000495083
Zhang Y, Han B, He Y, Li D, Ma X, Liu Q, Hao J (2017b) MicroRNA-132 attenuates neurobehavioral and neuropathological changes associated with intracerebral hemorrhage in mice. Neurochem Int 107:182–190. https://doi.org/10.1016/j.neuint.2016.11.011
Zhao M, Wang J, Xi X, Tan N, Zhang L (2018) SNHG12 promotes angiogenesis following ischemic stroke via regulating miR-150/VEGF pathway. Neuroscience 390:231–240. https://doi.org/10.1016/j.neuroscience.2018.08.029
Author information
Authors and Affiliations
Contributions
Zhirong He conceived, designed, and revised the current study. Yanyan Zhao, Yongxia Zhu, Weihua Wang, Xin Liu, and Fen Lu analyzed the data. Zhirong He wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, Z., Zhao, Y., Zhu, Y. et al. Interfering TUG1 Attenuates Cerebrovascular Endothelial Apoptosis and Inflammatory injury After Cerebral Ischemia/Reperfusion via TUG1/miR-410/FOXO3 ceRNA Axis. Neurotox Res 40, 1–13 (2022). https://doi.org/10.1007/s12640-021-00446-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00446-7