Abstract
Aside from the respiratory distress as the predominant clinical presentation of SARS-CoV-2 infection, various neurological complications have been reported with the infection during the ongoing pandemic, some of which cause serious morbidity and mortality. Herein, we gather the latest anatomical evidence of the virus’s presence within the central nervous system. We then delve into the possible SARS-CoV-2 entry routes into the neurological tissues, with the hematogenous and the neuronal routes as the two utmost passage routes into the nervous system. We then give a comprehensive review of the neurological manifestations of the SARS-CoV-2 invasion in both the central and peripheral nervous system and its underlying pathophysiology via investigating large studies in the field and case reports in cases of study scarcity.
Similar content being viewed by others
Introduction
In 2019, novel cases of pneumonia of unknown cause in Wuhan, China, were reported (Disease outbreak news 2019) (Disease (Disease outbreak news 2019), and later that year, the underlying pathogen was designated as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Declared by World Health Organization (WHO), the coronavirus disease 2019 (COVID-19) caused a global pandemic, posing a major public health crisis (World Health Organization 2019; Wuhan Municipal Health Commission 2020). As of December 2020, over 67 million cases with SRAS-CoV-2 infection have been reported, with more than a million and a half casualties worldwide (World Health Organization 2020).
SARS-CoV-2 can infect all age groups, regardless of gender, ethnicity, and general health status. Direct contact, aerosol, and droplet transmission are the three main routes of SARS-CoV-2 transmission (Mehraeen et al. 2020). Although the pulmonary system is its primary entry site, a myriad of non-respiratory clinical symptoms alongside cumulating anatomical evidence has demonstrated the virus’s potential to invade multiple organs throughout the body including the nervous system.
A thorough grasp over the interplay between the virus and its accompanying disease and the nervous system is needed to identify new diagnostic measurements, further unveil the disease’s mechanism of action, and ultimately find ways to alleviate the neuro-pathophysiological complications of COVID-19 and discover novel therapeutic targets and strategies.
To do so, in this article, we initially depicted the routes the virus exploits to reach the central nervous system (CNS). A multitude of studies was gathered to illustrate the anatomical traces of the virus’s presence within the CNS. We then comprehensively analyzed the neurological complications of COVID-19 and related probable mechanisms within the CNS and the peripheral nervous system (PNS) by reviewing the studies and case reports submitted until the date of preparing this manuscript. We mainly focused on the underlying physiopathology via evaluating the large studies in the field. However, case reports were reported as well when no large study was available on a specific topic.
Anatomical Manifestations of SARS-CoV-2 Presence in the CNS
Generally speaking, the coronaviruses’ entry mechanism includes binding of the spike (S) protein to a particular cellular receptor. Subsequently, cellular proteases would prime the S protein. Like its precursor SARS-CoV, cellular entry SARS-CoV-2 initiates by binding of receptor binding domain (RBD) portion of the virus’s Spike (s) protein to angiotensin-converting enzyme 2 (ACE-2) receptor leading to direct and indirect mechanisms of SARS-CoV-2 neurotoxicity (Tancheva et al. 2020). The virus entry to the cell requires S priming, and it is mediated by the serine protease TMPRSS2. TMPRSS2 cleaves S protein at the S1/S2 and the S2’ sites. The viral and cellular membranes are then fused through a process driven by the S2 subunit, and the virus enters the cell (Hoffmann et al. 2020; Sungnak et al. 2020; Wang et al. 2008).
As reported previously for other coronavirus infection, increasing evidence unravel the presence of SARS-CoV-2 in multiple CNS loci, concomitant with various neuropathological manifestations. Vascular events counted as one of the most prevalent manifestations of SARS-CoV-2 presence within the CNS (Dmytriw et al. 2020; Dorche et al. 2020; Hamidianjahromi and Mowla 2021; Mowla et al. 2020a, b, c; Shahjouei et al. 2021, 2020; Shakibajahromi et al. 2020b; Sharifian-Dorche et al. 2020; Vahabizad et al. 2020). Multiple postmortem anatomical dissections revealed thrombosis and microthrombi (six out of 20 (Bryce et al. 2020) and four out of 12 (Meinhardt et al. 2020)), concomitant with ischemia and subsequently acute infarctions within the CNS. In another study, three out of 17 autopsied cases had focal ischemic necrosis was observed (Remmelink et al. 2020). In the histopathological examination of 18 COVID-19 deceased patients’ brain specimens, Solomon et al. (2020) reported atherosclerosis in 14 patients (Solomon et al. 2020). It was proposed that the thromboembolic events provoked by SARS-CoV-2 infection may represent a secondary anti-phospholipid antibody syndrome (Cavalli et al. 2020).
Multiple studies reported intracerebral hemorrhage as a neurological manifestation of SARS-CoV-2 CNS infection. ACE2 expression on the cerebrovascular endothelial cells and its interaction with the virus is speculated to cause a large intracerebral hemorrhage in a 79-year-old patient, accompanied by intraventricular and subarachnoid hemorrhage. Virus-mediated activation of ACE-2 is hypothesized to disrupt the cerebral auto-regulation and elevate the neurogenic hypertension (Mondal et al. 2020). Eight of 11 COVID-19 non-survivors’ brain autopsies displayed cerebral hemorrhage or hemorrhagic suffusion (Remmelink et al. 2020). Hemorrhagic white matter lesions were present throughout the cerebral hemispheres of another deceased patient with surrounding axonal injury and macrophages (Reichard et al. 2020). Three of six autopsies displayed massive intracranial hemorrhage with diffuse petechial hemorrhage in the entire brain (von Weyhern et al. 2020).
Although several studies reported no signs of encephalitis within the patients’ CNS, Moriguchi et al. (2020) were first to report encephalitis by detecting the viral RNA within the CSF (Moriguchi et al. 2020). Focal parenchymal infiltrate of T-lymphocyte was detected in two out of 20 samples, indicating focal encephalitis (Bryce, et al. 2020). Alongside radiographic reports of acute hemorrhagic necrotizing encephalitis (AHNE) and acute disseminated encephalomyelitis (ADEM), Reichard et al. (2020) reported widespread hemorrhagic white matter lesions resembling AHNE and scattered clusters of macrophages, a range of associated axonal injury, and a perivascular ADEM-like appearance in the subcortical white matter (Poyiadji et al. 2020; Reichard et al. 2020). AHNE was also observed in the brain MRI accompanied by mild lymphocytosis within the CSF in a critically ill COVID-19 patient (Ghosh et al. 2020). SARS-CoV-2-induced encephalitis is pathophysiologically explained by the immunologic response causing inflammatory injury and edema, as it ameliorates when the patient responds to the supportive therapeutic regimen (Wu et al. 2020; Ye et al. 2020). Lymphocytic pan-encephalitis is documented in a study on six postmortem brain specimens (von Weyhern et al. 2020).
Perivascular and interstitial encephalitis were reported in six brainstem autopsies. These events were accompanied by axon degeneration and neuronal cell loss in the dorsal motor nuclei of the vagus nerve, dorsal raphe nuclei, CN V, fasciculus longitudinalis medialis, and nucleus tractus solitarii. The direct viral invasion via the peripheral nerves of the respiratory network into the medulla oblongata and particularly the medullary cardiorespiratory center is proposed as the underlying culprit of the respiratory complications of COVID-19 patients (Mehta et al. 2020). Anatomical dissection of the CNS of a 54-year-old male revealed viral infection and the resulting tissue damage involving the neurons, glia, nerve axons, and myelin sheath in the medulla oblongata, albeit less severe than those observed in olfactory nerves, justifying the specific respiratory dyssynergia observed in some patients (Bulfamante et al. 2020). Diffusive swelling and symmetrical hemorrhagic lesions were detected in the medulla oblongata of another COVID-19 non-survivor, serving as another case of necrotizing hemorrhagic encephalopathy afflicting the brain stem (Dixon et al. 2020).
Hypoxia or anoxia injury to the nervous system is one of the most critical complications in COVID-19 patients particularly among those with severe decreased O2 saturation. Via microscopic examination, all of 18 brain specimens possessed acute hypoxic injury in the cerebrum and cerebellum, with loss of neurons in the hippocampus, cerebral cortex, and cerebellar Purkinje cell layer, but no thrombi or vasculitis was observed. Alongside the fact that immunohistochemical analysis of the neurons, glia, endothelium, or immune cells in neither of specimens stained positive for SARS-CoV-2, Solomon et al. (2020) concluded that neuropathological manifestations in COVID-19 patients predominantly rely on hypoxia-related complications rather than encephalitis or other specific brain changes referable to the virus (Solomon et al. 2020).
Direct evidence of the viral presence in the brain tissues and endothelial cells are reported. From postmortem examination of a SARS‐CoV‐2‐infected patient, viral particles were detected in the capillary endothelial cells individually and in small vesicles, pinpointing the virus’s transcellular passage across the brain microvascular endothelial cells into the neural niche, as the vascular endothelial cells express ACE2. The pleomorphic spherical viral‐like particles were evident in the neural cell bodies as distended cytoplasmic vacuoles in transmission electron microscopy (Paniz-Mondolfi et al. 2020). In another anatomical dissection of COVID-19 demised patients at Mount Sinai Hospital in New York City, brain parenchyma is reported to express ACE2. As an alternative to conspicuous inflammation, the neuronal impairment caused by hypoxia is claimed to be the cause of the neuropsychiatric symptoms (Bryce et al. 2020). Cerebellum, cornea, conjunctiva, oral mucosa, and gyrus rectus are among the other brain regions in which the presence of the viral RNA was reported (Bulfamante et al. 2020; Meinhardt et al. 2020).
SARS-CoV-2 Entrance Routes into the CNS
As seen with the other respiratory viruses, including SARS-CoV-1 and MERS-CoV, neuropathological characteristics of SARS-CoV-2 shed light on its neuro-invasiveness and neuro-virulence (DosSantos et al. 2020; Li et al. 2016; Netland et al. 2008). Specialized glial cells known as olfactory ensheathing cells (OECs) are proposed to mediate ACE2-independent virus dissemination via the release of viral particle-carrying extracellular vesicles toward the olfactory receptor neurons axons (Yavarpour-Bali et al. 2020). However, SARS-CoV-2 is hypothesized to invade the CNS through two major pathways, the hematogenous and the neuronal routes.
Hematogenous Route
It is well established that SARS-CoV infects the cerebrovascular endothelial cells and passes through the blood–brain barrier (BBB) into the CNS and also invades the CNS through shedding inside the blood cells (Gu et al. 2005; Guo et al. 2008). Like its predecessor, accumulating evidence demonstrates the SARS-CoV-2 passage through the blood into the CNS. Through the blood dissemination, the viral particles reach to and enter the cerebral capillary endothelial cells via binding to their predominantly expressed ACE2 receptors. Following posing substantial damage, the virus is released into the extracellular fluid of the central nervous system where neurons reside (Fig. 1a) (Mondal et al. 2020).
Hematogenous route of SARS-CoV-2 entry into the CNS. (a) SARS-CoV-2 is believed to pass the blood–brain barrier from the CSF into the brain tissue. Cerebral capillary endothelial predominantly express ACE2, and after they drastically damage their host cells, the viral particles are released into the extracellular fluid of the central nervous system where neurons are present. (b) The epithelium of BCSFB across the choroid plexus in the ventricles highly expresses ACE-2, mediating the virus passage into the CNS. (c) SARS-CoV-2 utilizes the Trojan horse mechanism to enter the CNS by hiding into the blood leukocytes. Other factors including Trojan horse mechanism further increases the BBB permeability, assisting the viral dissemination into the CNS via the immune cells
ACE2 is also present ubiquitously on the epithelial cells of the blood–cerebrospinal fluid barrier (BCSFB) across the choroid plexus in the ventricles, and SARS-CoV-2 can cross this barrier to reach the CNS since the viral RNA has been recorded in the CSF of a COVID-19 patient (Fig. 1b) (Desforges et al. 2019; Moriguchi et al. 2020).
Alternatively, since blood leukocytes, including lymphocytes, granulocytes, and monocytes, express ACE2, their infection via SARS-CoV-2 mediates viral passage through the BBB and the virus’s entrance into the CNS, a process dubbed “Trojan horse mechanism.” Moreover, the BBB’s permeability is widely promoted via the heightened systematic inflammatory mediators, which in turn facilitates the viral dissemination into the CNS via the immune cells (Fig. 1c) (Desforges et al. 2014; Gu et al. 2005; Trojanowicz et al. 2017).
Neuronal Pathways
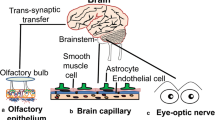
Multiple viral pathogens, including herpes simplex virus and herpes zoster, have demonstrated trans-synaptic propagation into the CNS or other nervous centers (DaSilva and DosSantos 2012; DosSantos et al. 2020). Two major mechanisms have been proposed for the nervous system-dependent entry of SARS-CoV-2 into the CNS, either through the transcribral route or via the neuronal retrograde dissemination. Upon viral infection through the nose, the olfactory epithelial cells, which express ACE2 and TRPMSS2, are ruptured, and heavy loads of the viral particles are released into the olfactory bulb through the cribriform plate. The virus is then transported into the various regions within the CNS via the CSF (Baig and Sanders 2020). Distinct neurological manifestations resulting from the damaged olfactory epithelium, including anosmia or hyposmia, corroborates this viral entry route (Fig. 2) (Baig et al. 2020; Mao et al. 2020).
Neuronal pathways of SARS-CoC-2 entry into the CNS. (a) Through the transcribral route, the virus enters and ruptures the ACE2 and TRPMSS2-expressing olfactory epithelial cells. The viral particles are then released into the olfactory bulb through the cribriform plate, and then they are transported into the various regions within the CNS via the CSF. (b and c) Nasal respiratory epithelium, small intestine and colon epithelial cells, and epithelial cells of the oral mucosa express high levels of ACE2. The virus can travel back from the periphery into the nervous centers through the retrograde spread via trans-synaptic transfer
Alternatively, the virus can travel back from the periphery into the nervous centers through the retrograde spread via trans-synaptic transfer. Nasal respiratory epithelium, small intestine and colon epithelial cells, and epithelial cells of the oral mucosa express high levels of ACE2. Upon entry to the axons, the virus transfers backward toward the neural bodies of nerves. Peripheral neurons including the vagal nerves, enteric nerves, olfactory nerves, and trigeminal nerves and in general, the majority of the cranial nerves, are assumed to mediate viral passage through the retrograde mechanism into various CNS centers. These centers, including the medullary cardiorespiratory centers, are where respiratory complications may stem from (Fig. 2) (Ferreira et al. 2020; Lamers et al. 2020; Liang et al. 2020; Zhou et al. 2020).
Viral Infection of Cells Within the CNS
Upon the virus’s entry into the CNS, it enters the nervous cells via ACE2 expressed on their membrane. Brain transcriptome databases analysis revealed that ACE2 is highly expressed in some brain regions, such as the substantia nigra and lateral ventricles. The cell-type distribution analysis revealed ACE2 presence in both excitatory and inhibitory neurons and also some non-neuron cells including oligodendrocytes and astrocytes, in posterior cingulate cortex and middle temporal gyrus (Chen et al. 2020).
Several studies specifically delineated the viral dissemination within the neurons. Through transmission electron microscopy of deceased patients’ brain specimens, Paniz-Mondolfi et al. (2020) recorded the blebbing of the viral‐like particles coming in/out of the endothelial wall and their subsequent release from the brain microvascular endothelial cells into the neural niche. Distended cytoplasmic vacuoles containing enveloped viral particles were also observed within the neural cell bodies (Desforges et al. 2014).
New Potential Drugs for COVID-19 Therapies
According to suggested underlying pathophysiology, possible novel drug options for the treatment of SARS-CoV-2 infection were predicted including anti-proliferative drugs, such as mitogen-activated protein kinase, serine-threonine kinase, mammalian target of rapamycin, and I kappa B kinase inhibitors (Fagone et al. 2020). For example, baricitinib as an anti-rheumatoid JAK inhibitor proposed with well-tolerated brain penetration may reduce neurological deficits arising from SARS-CoV-2 infection (D’Silva and Wallace 2021; Richardson et al. 2020).
Clinical Consequences of Nervous System Following COVID-19 Infection
With the spread of SARS-CoV-2 worldwide, a myriad of neurological complications has been reported within the PNS and CNS (Elmashala et al. 2020; Tancheva et al. 2020). In some cases, neurological manifestations are the presenting symptoms while typical pulmonary symptoms are absent. While occurring in patients regardless of the severity of the disease, neurological manifestations are more frequent in critically ill patients (Mao et al. 2020). A significant correlation exists between the severity of the neurological complications and the severity of the disease, cumulatively leading to a higher rate of mortality (Merkler et al. 2020; Yaghi et al. 2020). Thus, clinicians should be observant of these manifestations, particularly in critically ill patients. The following sections discuss a thorough review of the neurological manifestations of the SARS-CoV-2 infection in both CNS and PNS (Table 1).
Central Nervous System Complications
Compared to the PNS complications, CNS complications are more prevalent in sicker patients and may worsen the overall outcome of the disease (Mao et al. 2020). Impaired consciousness, encephalopathy, cranial nerve palsy, cerebral venous sinus thrombosis, ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage, headache, seizure, meningitis/encephalitis, and myelitis are the main CNS complications following the SARS-CoV-2 infection.
Acute Confusional State
In acute confusional state consciousness impaired, including both level and content of consciousness (Pezzini and Padovani 2020). Altered mental status might lead to longer hospitalization and poor prognosis (Liotta et al. 2020). In a study by Helms et al. (2020) in France, 140 COVID-19 patients with ARDS enrolled, of which 118 (84.3%) developed delirium and 88 (69.3%) presented with agitation (Helms et al. 2020). In the USA, among 250 patients included in Chachkhiani et al. (2020) study, 19 patients (8%) reported altered mental status at presentation, and 73 (29%) developed altered mental status during hospitalization (Chachkhiani et al. 2020). In another study in the USA, 27 out of 76 patients with COVID-19 displayed neurological symptoms. Altered mental status was observed in 26 (96%) (Scullen et al. 2020).
In Turkey, Karadaş et al. (2020) investigated neurological manifestations in 239 patients with SARS-CoV-2 infection. Twenty-three (9.6%) patients developed impaired consciousness, being the most prevalent neurological finding following headache (Karadaş et al. 2020). Mao and his colleagues conducted a study in China on 214 patients, of whom 16 (7.5%) developed impaired consciousness, with 13 of them having severe SARS-CoV-2 infection (Mao et al. 2020). Three out of 73 COVID-19 patients with neurological manifestations studied by Chougar et al. (2020) in France, developed coma (Chougar et al. 2020). Among 101 patients participating in Lovell et al. (2020) study in the UK, 24 individuals developed delirium, 36 drowsiness, and 43 agitation (Lovell et al. 2020).
Encephalopathy
Encephalopathy associated with COVID-19 is mainly due to hypoxia, metabolic dysregulation following organ failure, and cytokine release that may lead to neuronal dysfunction named cytokine sickness (Iadecola et al. 2020). Hence, it is more frequent in patients with severe disease or associated comorbidities (Pezzini and Padovani 2020). Among 12,601 hospitalized patients that were diagnosed with COVID-19 from January 20 to June 29, 2020, in the USA, 8.7% (1092 patients) developed acute encephalopathy. Compared to the patients who did not develop encephalopathy, patients with encephalopathy were older, had higher prevalence of comorbidities, were more likely to need intubation, and had higher 30-day mortality rate (Shah et al. 2020).
From March 1, 2020, to April 30, 2020, fifty SARS-CoV-2 infected confirmed cases with neurological symptoms were identified in a medical center in Chicago. Thirty patients had encephalopathy, which was the first presentation in 12 (40%) (Pinna et al. 2020). In another study based in the USA, 27 of 76 SARS-COV-2-positive patients showed neurological manifestation, of whom 20 were diagnosed with COVID-19-associated encephalopathy and two with COVID-19-associated acute necrotizing encephalopathy (Scullen et al. 2020).
In addition, several cases of posterior reversible encephalopathy syndrome (PRES) and AHNE have been reported (Anand et al. 2020; Ghosh et al. 2020; Cariddi et al. 2020).
Cranial Nerve Palsy
The etiology of the isolated cranial nerve palsy in COVID-19 patients that has been frequently reported is still debated. Both direct and indirect neuroinflammatory and autoimmune mechanisms associated with viral injury have been proposed to be involved in the pathophysiology of the disease (Vonck et al. 2020). In the USA, Pranusha Pinna and her colleagues reported neurological manifestations of 50 COVID-19 patients. Three of them experienced isolated facial palsy, and in one of them, the neurological symptom preceded COVID-19 flu-like symptoms (Pinna et al. 2020). In another study in the USA, Falcone and his team presented a case of abducens nerve palsy in a 30-year-old man with COVID-19 who developed acute, binocular, horizontal diplopia. His visual symptoms initiated 3 days after upper respiratory illness manifestations. An atrophic left lateral rectus muscle was revealed in his orbit MRI imaging (Falcone et al. 2020). In a similar study by Alice Faucher and her team in France, a 21-year-old man was reported to have had binocular horizontal diplopia after discharge from the hospital following COVID-19 illness. He was further diagnosed with partial paralysis of the left third cranial nerve which resolved spontaneously (Faucher et al. 2020).
Cerebral Venous Sinus Thrombosis
As SARS-CoV-2 infection has been associated with hypercoagulable state, cerebral venous sinus thrombosis (CVST) occurrence is quite probable. Since CVST can lead to more severe complications if not diagnosed in time, it should be considered as a probable differential diagnosis in COVID-19 patients presenting with headache, findings suggestive of elevated ICP and decreased level of consciousness (Shakibajahromi et al. 2020a). Twenty-two patients with COVID-19 developing acute cerebrovascular pathologies in the USA were included in a study by Ahmad Sweid and his colleagues of whom, two was shown to have dural sinus thrombosis (Sweid et al. 2020). Among 73 patients reported in Chougar study from France, extensive deep cerebral venous thrombosis complicated by hemorrhagic venous infarction was diagnosed in a 72-year-old male ICU patient (Chougar et al. 2020). A case of postpartum extensive CVST of multiple deep and superficial veins was reported by Taylor et al. (2020) in the USA (Taylor et al. 2020).
Stroke
The etiology of cerebrovascular disorders (CVDs) associated with SARS-CoV-2 infection is proposed to be multifactorial, including dysregulation of neuroinflammation, vasodilation, oxidative stress, and thrombotic reaction (Divani et al. 2020). As CVD might lead to poor outcomes in patients with COVID-19 (Pranata et al. 2020), physicians should be more attentive about timely diagnosis and treatment of acute ischemic stroke during the pandemic (Li et al. 2020). The thrombotic effect of COVID-19 has been reported frequently (Belani et al. 2020; Beyrouti et al. 2020; Chougar et al. 2020; Díaz-Pérez et al. 2020; Khan et al. 2020; Majidi et al. 2020; Oxley et al. 2020; Paterson et al. 2020; Sweid et al. 2020; TunÇ et al. 2020; Usman et al. 2020). Among 239 COVID-19 patients who had participated in Karadaş et al. (2020) study in Turkey, nine (3.7%) patients developed cerebrovascular events, two hemorrhagic, and seven ischemic strokes (Karadaş et al. 2020). A single-center study of 221 consecutive hospitalized patients with COVID-19 in China reported that 11 (5%) suffered acute ischemic stroke, one (0.5%) cerebral venous sinus thrombosis, and one (0.5%) cerebral hemorrhage (Li et al. 2020). In another study in China, of 214 patients with COVID-19 enrolled in Mao et al. (2020) study, 78 (36%) patients had some types of neurological manifestations. Central nervous system complications were recorded in 53 patients (24.8%). Acute cerebrovascular diseases were reported in six patients and were more prevalent in severe COVID-19 infection compared to mild disease (5 versus 1) (Mao et al. 2020). In France, Hautecloque and his colleagues enrolled 674 patients with confirmed COVID-19 in their study. Seven patients had ischemic stroke in a mean duration of 14.5 days after the flu-like symptoms onset. None needed to be admitted to the ICU. The predominance of cardioembolic mechanisms, high levels of inflammatory markers, and procoagulant state were reported in these patients.
In the USA, Pinna et al. (2020) evaluated 50 patients with COVID-19 seen by their neurology service. Ten patients suffered an acute ischemic stroke, and half of them had neurological manifestations as their first symptom (Pinna et al. 2020).
Intracerebral Hemorrhage
Intracerebral hemorrhage ICH has been reported in COVID-patients, leading to poor prognosis and high mortality rate (Cheruiyot et al. 2020). Direct endothelial injury and indirect pathway through cytokine release or coagulation cascade activation have been proposed as the potential underlying mechanisms (Benger et al. 2020).
Following SARS-CoV-2 S protein binding to ACE-2 and its entrance into the cell, ACE-2 and angiotensin 2 (AT-2) pathways may be downregulated (Zou et al. 2020). This downregulation leads to unbalanced over-activation of AT-1 and inhibition of AT-2, which in turn results in increased vascular permeability and acute organ injury (Iroegbu et al. 2020). As seen in four out of 20 demises from severe SARS-CoV-2 infection, this mechanism is speculated to underlie the disruption in brain capillary endothelium permeability and integrity (Coolen et al. 2020). These events may consequently lead to changes in cerebral blood flow autoregulation, and the risk of cerebral hemorrhage or ischemia would be elevated (Scoppettuolo et al. 2020; Sharifi-Razavi et al. 2020).
Among 214 patients involving in Mao et al. (2020) study in China, 78 patients had neurological symptoms. Neurological manifestations were more frequent in patients with severe disease. Six patients developed cerebrovascular disease: four ischemic stroke and one hemorrhagic stroke who died due to respiratory failure (Mao et al. 2020). Twenty-six COVID-19 patients with neurological complications were detected by Taylor and his colleagues between March 1 and May 24, 2020, from three academic tertiary care hospitals in the USA. Seven patients suffered intracranial hemorrhage: three hemorrhagic conversion of ischemic stroke, one intracerebral hemorrhage, one subarachnoid hemorrhage, and two hemorrhagic tumors (Taylor et al. 2020). Gogia et al. (2020) reported a 75-year-old female with COVID-19, who presented with hypoxia and was intubated. A few days later, she underwent a brain computed tomography (CT) scan due to mental status changes which revealed a left-sided acute subdural hematoma, causing left to right midline shift, a large left temporal intraparenchymal associated with subarachnoid hemorrhage and transtentorial herniation, leading to death (Gogia et al. 2020). In Kuwait, Savić et al. (2020) presented a 13-year-old case with severe intracerebral hemorrhage as the earliest manifestation of COVID-19 due to ruptured cerebral pseudoaneurysm of the left middle cerebral artery (Savić et al. 2020). In Iran, Sharifi-Razavi et al. (2020) reported a 79-year-old male COVID-19 patient with fever and cough for three days who referred to ED due to loss of consciousness. Brain CT scan showed a massive intracerebral hemorrhage in the right hemisphere, accompanied by intraventricular and subarachnoid hemorrhage (Sharifi-Razavi et al. 2020).
Headache
Headache is a common symptom in viral infections. It has been reported in COVID-19 patients with a high prevalence (Mutiawati et al. 2020). However, several characteristics of headache might differ in COVID-19 patients. The headaches are more common in males. They are bilateral, long lasting, and resistant to analgesics, according to a study conducted by Uygun et al. (2020) in Turkey (Uygun et al. 2020). Among 221 COVID-19 patients from Zhongnan Hospital of Wuhan, China, headache was reported in 17 patients (7.7%), and four of them had severe COVID-19 illness (Zhang et al. 2020). In Spain, 576 COVID-19 patients recruited in García-Azorín et al. (2020) study, 130 (22.6%) had headache. One hundred four of them were studied. Sixty (57.7%) mentioned a prior history of headache syndromes (migraine in 17/104 (16.3%), tension-type headache in 30/104 (28.8%)). Twenty-seven (26.0%) patients described headache as their first COVID-19 symptom (García-Azorín et al. 2020). Seventy percent of the 1420 patients included in a study in France experienced headache (Lechien et al. 2020b). In another study with 108 participants in Italy, 46 (43%) reported headache. In another study in Spain, Trigo and his colleagues described 576 COVID-19 patients with a mean age of 67.2, 43.3% female. One hundred and thirty-seven (23.7%) had headache, and 24 of them experienced it as their first COVID-19 symptom. Headache was associated with lower mortality risk. Headache was more frequent in female sex, in patients with prior history of headache, anosmia, myalgia, and fever, according to this study (Trigo et al. 2020).
Suleyman et al. (2020) reported that following prodromal symptoms of COVID-19 in 463 patients, headache presents in 74 (16%) of them. Twenty-eight (25.9%) were discharged home, and 46 required hospital admission. Headache was more prevalent among patients in the regular floors compared to the patients in intensive care units (Suleyman et al. 2020). In Turkey, Toptan et al. (2020) published a report of 13 patients with mild COVID-19 infection who developed headache. Three of them had headache as their first presenting symptom. The mean age of the patients was 40.2 ± 11. Their headache was moderate to severe in intensity, throbbing, holocranial with a predominant focus in bilateral frontal and temporal areas. Seventy percent of patients recovered within 3 days. Photophobia and/or phonophobia were often seen with headache, and they were troublesome in patients with a history of migraine (Toptan et al. 2020). Headache was shown to be associated with poor outcome according to another report from Spain (Gil-Rodrigo et al. 2020), and it was more prevalent in patients with the history of headache (81% versus 36%) (Vacchiano et al. 2020).
Seizure
Seizure is a rare complication of COVID-19 except in severe cases (Hogan et al. 2020; Lu et al. 2020a). It might be a consequence of hypoxia, cytokine release, cerebrovascular events, or fever (Vohora et al. 2020). A total of 257 patients were admitted to the University Medical Center New Orleans (UMCNO) for COVID-19 during March 2020. Neurological symptoms were recorded at presentation and during hospitalization. One patient presented with seizure, and 10 patients had seizure during the hospital cure according to Chachkhiani et al. (2020). Furthermore, patients who experienced seizure had higher odds of demanding intubation (Chachkhiani et al. 2020). In a study from the USA, seizure was one of the most common neurological symptoms among 50 patients with COVID-19 and was more frequent in patients who presented with COVID pulmonary symptoms rather than those who initially presented with neurological manifestations (Pinna et al. 2020). In France, Chougar and his team reported a retrospective study of 73 COVID-19 patients with neurological manifestation. Ten (13.7%) patients had seizure (Chougar et al. 2020).
Meningitis/Encephalitis
Encephalitis is the inflammation of the brain tissue. Viral infections are the leading cause, followed by autoimmune conditions. The inflammation of the meninges is called meningitis. Similar to encephalitis, the most common cause of meningitis is viral infection as well.
The first case of meningitis associated with COVID-19 in Japan was reported by Moriguchi et al. (2020). A 24-year-old man presented with flu-like symptoms. After 9 days of symptom onset, he was brought to hospital with loss of consciousness (GCS = 6). He developed a transient generalized tonic-clonic seizure for about a minute during emergency department admission. SARS-CoV-2 RNA was detected in his CSF sample, and his brain MRI revealed hyperintensity along the wall of the right lateral ventricle and hyperintense signal changes in the right mesial temporal lobe and hippocampus, suggesting the likelihood of SARS-CoV-2 meningitis (Moriguchi et al. 2020).
In the UK, Khoo et al. (2020) described a 65-year-old woman with presumed Alzheimer’s disease who presented with generalized myoclonus, ocular flutter with convergence spasm, and acquired hyperekplexia. She had fever and cough the week before she developed neurological symptoms. Her SARS-CoV-2 RT-PCR was positive. She was finally diagnosed with postinfectious immune-mediated encephalitis and treated with corticosteroids (Khoo et al. 2020).
Bernard-Valnet and his colleagues reported two patients with COVID-19 and neurological manifestations in Switzerland. The first patient was a 64-year-old female with acute psychotic symptoms after 5 days of flu-like symptoms. She developed seizure during hospitalization. Her lumbar puncture revealed viral meningoencephalitis. The second one was a 67-year-old woman who presented with an intense wake-up headache, 17 days after COVID-19 diagnosis. She further developed decreased consciousness, aggression, left-sided hemianopia, and sensory hemineglect. Her lumbar puncture displayed lymphocytic pleocytosis. Both patients were discharged in good health after antiviral treatment (Bernard-Valnet et al. 2020). Similarly, Parsons et al. (2020) reported a 51-year-old woman with COVID-19 in the USA, who was referred to a tertiary hospital with coma, impaired unilateral oculocephalic response, and left hemiparesis. Based on her brain MRI findings, CSF, and clinical course, acute disseminated encephalomyelitis (ADEM) diagnosis was made. She became oriented after methylprednisolone and IVIG administration (Parsons et al. 2020). Moreover, cytotoxic lesions of the corpus callosum (CLOCCs) were reported in several studies (Forestier et al. 2020; Gaur et al. 2020; Moreau et al. 2020).
Myelitis
Myelitis is the inflammation of the spinal cord which leads to neurological symptoms. Several cases were reported with myelitis as a manifestation of COVID-19.
In a study by Munz et al. (2020), a 60-year-old man admitted to a hospital in Germany with respiratory symptoms of COVID-19, developed neurological manifestations 3 days after discharge. He complained of bladder dysfunction, progressive lower limbs weakness, hypesthesia below the T9 level, and moderate spasticity of the legs. Bilateral Babinski’s signs were shown on neurological examination. MRI of the spine showed T2 signal hyperintensity of the thoracic spinal cord at T9 level suggestive of acute transverse myelitis. He received methylprednisolone and improved (Munz et al. 2020). In India, Chakraborty and his colleagues presented a 59-year-old female with 4 days of fever and progressive ascending flaccid paraplegia who initially had a negative RT-PCR test result for SARS-CoV-2. MRI T2-weighted imaging of the spine revealed hyperintensity in the spinal cord at T6–T7 level, suggestive of myelitis. Methylprednisolone was started, and her neurological symptoms improved. However, she passed away later due to sudden-onset respiratory distress, and her repeated SARS-CoV-2 RT-PCR test was positive (Chakraborty et al. 2020). Sotoca and Rodríguez-Álvarez (2020) presented a 69-year-old female with neck pain, imbalance, and motor weakness and numbness in the left arm after 8 days of fever and cough. Her spine MRI revealed T2-hyperintensity extending from the lower medulla oblongata to C7, involving most of the cord with diffuse patchy enhancing lesions, suggesting acute transverse myelitis. She showed initial improvement following methylprednisolone administration but worsened after a few days, and her new spinal MRI displayed transversally and caudally progression down to T6 level with similar enhancement patterns and a new area of central necrosis at the T1 level with peripheral enhancement. Plasma exchange was performed, and she showed some improvement. Her postplasmapheresis spinal MRI demonstrated a substantial decrease in myelitis extension and enhancement, but central necrosis at the C7-T1 level remained unchanged. Acute necrotizing myelitis was diagnosed (Sotoca and Rodríguez-Álvarez 2020).
Peripheral Nervous System Complications
Compared to CNS symptoms, PNS symptoms are less severe (Azhideh 2020), including hyposmia/anosmia, hypogeusia/ageusia, neuropathies, myopathies, and neuromuscular junction disorders.
Smell and Taste Alteration
Alteration in smell and taste such as anosmia, hyposmia, hypogeusia, or dysgeusia have been reported with COVID-19 (Altin et al. 2020; Boddington et al. 2020; D'Ascanio et al. 2020; Gómez-Iglesias et al. 2020; Liguori et al. 2020; Ortiz-Brizuela et al. 2020; Paderno et al. 2020; Pinna et al. 2020; Salepci et al. 2020; Spadera et al. 2020). Although these symptoms might occur with other respiratory infections, it has been proven to be more prevalent in SARS-COV-2 infection compared to influenza virus infection (Beltrán-Corbellini et al. 2020). As such, it can be a helpful distinguishing symptom for the diagnosis of COVID-19. Whether chemosensory impairment predicts mild or severe form of disease is a matter of debate (Neto et al. 2020; Giacomelli et al. 2020; Yan et al. 2020). In France, 417 mild-to-moderate COVID-19 patients were enrolled in a study by Lechien et al. (2020a, b); 85.6% and 88.0% of the patients had olfactory and gustatory dysfunctions, respectively. Of the cases, 11.8% experienced olfactory dysfunction before the other symptoms. According to this study, females were more likely to have olfactory and gustatory dysfunctions than males. Overall, 79.6% of the patients with olfactory dysfunction were anosmic, 20.4% were hyposmic, 12.6% were phantosmic, and 32.4% were parosmic. Among patients with gustatory dysfunction, reduced/discontinued or distorted ability to taste flavors affected 78.9% and 21.1% of patients, respectively (Lechien et al. 2020a). In a cohort performed by Foster and his colleagues in the USA, 198 (20.9%) out of 949 patients with COVID-19 experienced smell loss. Patients with a history of preexisting smell dysfunction, allergic rhinitis, or chronic rhinosinusitis, young age, female sex, and higher body mass index were more prone to acute smell loss (Foster et al. 2020). Yonker et al. (2020) enrolled 192 children, of whom 49 were confirmed to have COVID-19 in his study. Ten of them developed anosmia/hyposmia (20.4%), and three had dysgeusia (6.1%) (Yonker et al. 2020). Among 345 COVID-19 patients in Vaira et al. (2020) study in Italy, 256 patients reportedly had chemosensitive dysfunctions, 8.6% isolated olfactory disorders, 12.1% isolated taste disorders, and 79.3% combined chemosensitive disturbances (Vaira et al. 2020). In Lechien et al. (2020a, b) study involving 1420 COVID-19 patients in France, loss of smell and gustatory dysfunction were among common COVID-19 symptoms in 70.2% and 54.2% of the patients, respectively. According to this report, loss of smell was experienced by females more than males, young adults more than elderly were and was not associated with rhinorrhea and nasal obstruction (Lechien et al. 2020b). A total of 151 patients were enrolled in a cohort by Paderno and his colleagues in Italy. Olfactory dysfunction was reported in 83% of the patients (26% partial olfactory dysfunction and 74% anosmia), gustatory dysfunction in 30% as partial gustatory dysfunction, and 70% experienced ageusia. Thirty-day recovery of olfactory dysfunction and gustatory dysfunction rates were 87% and 82%, respectively. Grade of dysfunction at onset (total versus partial), gender, and presence or absence of nasal congestion were implicated in late recovery (Paderno et al. 2020).
Neuropathy
Eshak et al. (2020) reported a 72-year-old man admitted to a hospital in the USA with a week of fever and shortness of breath, diagnosed with COVID-19 infection. During hospitalization, he had labile blood pressures due to acute dysautonomia (Eshak et al. 2020). Pinna et al. (2020) also noted dysautonomia in six out of 50 COVID-19 patients who required neurology consultation (Pinna et al. 2020). Among 43 patients screened by Paterson et al. (2020) in the UK, one patient developed a brachial plexopathy 14 days after COVID-19 symptoms and was given corticosteroids, resulting in partial recovery (Paterson et al. 2020). Five out of 214 patients (2.3%) evaluated by Mao et al. (2020) in China experienced neuropathic pain. Four of them had severe COVID-19 infection (Mao et al. 2020). Malayala and Raza (2020) presented a case of a 29-year-old female with persistent vertigo due to COVID-19-induced acute vestibular neuritis (Malayala and Raza 2020). Acute polyradiculoneuritis was reported in a 51-year-old male with COVID-19 infection in Pfefferkorn et al. (2020) study in Germany (Pfefferkorn et al. 2020). Valliuddin and colleagues described a 61-year-old female with generalized weakness after a week of chills and rhinorrhea. Later she developed numbness and tingling in her hands and feet and progressed to severe weakness in her lower extremities bilaterally. Furthermore, the numbness had ascended to the abdomen, and she started having constipation and difficulty voiding. Her nasal swab test was positive for SARS-CoV-2. The cervico-thoraco-lumbar spine MRI showed extensive intramedullary disease throughout the entire length of the cervical spinal cord, with an ill-defined patchy hyperintense signal on the T2-weighted images along with mild enlargement of the caliber of the cord without contrast enhancement. Electromyography/nerve conduction study findings were compatible with a distal a motor, axonal-loss predominant, polyneuropathy impacting the lower extremities with evidence of ongoing active denervation. There was sparing of all sensory nerves tested. Elevated protein and albumin with a white-cell count of one per cubic millimeter were detected in her CSF (Valiuddin et al. 2020).
Guillain-Barré Syndrome
Guillain-Barré syndrome (GBS) or acute inflammatory polyradiculopathy is caused when the body’s immune system attacks peripheral nerves. GBS can occur at the time of viral infectious (parainfectious) or after that (postinfectious). COVID-19-related GBS is mostly categorized in the latter group. Considering the time, the underlying mechanism is most likely immune-mediated rather than direct injury. However, we cannot completely exclude the role of direct attack. At least 220 patients with SC2-GBS were reported in 95 papers that were collected by Finsterer J and Scorza FA. Age of these patients (reported in n = 215) ranged from 8 to 94 years. Gender (reported in n = 213) was male in 146 and female in 67. Latency between onset of COVID-19 and GBS (n = 194) ranged from − 10 to 90 days. SARS-CoV-2 was not positive in the CSF. Therapy of GBS (reported in n = 215) comprised intravenous immunoglobulins (n = 191), plasmapheresis (n = 15), steroids (n = 2), or no therapy (n = 7). Forty-one patients required artificial ventilation. Outcome (reported in n = 168) was assessed as complete recovery (n = 37), partial recovery (n = 119), or death (Finsterer and Scorza 2021). The first case of GBS associated with COVID-19 was reported by Zhao et al. (2020) in China (Zhao et al. 2020). Of 43 patients studied by Paterson et al. (2020) in the UK, 29 were SARS-CoV-2 RT-PCR positive and definite, eight probable, and six possible according to World Health Organization criteria. GBS was seen in seven of them. All were male, with the age range of 20 to 63. Three required intensive care unit care. Neurological symptoms onset ranged from one day before to 21 days after typical COVID-19 symptoms (mean = 13). Three of them had a positive nasopharyngeal RT-PCR test result for SARS-CoV-2. Treatment with IVIG was initiated, and two had complete recovery (Paterson et al. 2020; Riva et al. 2020).
Myalgia
Myalgia is a common symptom in COVID-19 similar to other viral infections (Boddington et al. 2020; Guan et al. 2020; Hong et al. 2020; Klopfenstein et al. 2020; Liguori et al. 2020; Mo et al. 2020; Rep 2020; Wang et al. 2020; Zayet et al. 2020) (2019, but is reported to be more severe and last longer) (Kucuk, et al. 2020). COVID-19 infection may exacerbate myalgia and fatigue for prolonged duration as compared to patients with other viral infections. Myalgia reflects generalized inflammation and cytokine response and can be the onset symptom of 36% of patients with COVID-19. However, it is not a prognostic factor for severity of the disease (Lippi et al. 2020). Almazeedi et al. (2020) conducted a cohort including 1096 patients in Kuwait. Seventy-five (6.5%) of them experienced myalgia. Of whom, four required ICU admission and two passed away (Almazeedi et al. 2020). Among a total of 1000 patients evaluated in Argenziano et al. (2020) study, 268 (26.8%) complained of myalgia (Argenziano et al. 2020). In another study, DeBiasi and associates screened 177 infected young adults and children with the median age of 9.6 years (range, 0.1–34.2 years). Myalgia was reported in 25 (14%) of them, among them 21/133 (16%) were non-hospitalized, 4 /44 (9%) were hospitalized, 2/35 (6%) were in non-critical care units and 2/9 (22%) were in critical care units (DeBiasi et al. 2020).
Myopathy
Hyperinflammation and metabolic pathways may affect muscles from hypo-excitability to necrosis (Versace et al. 2021). Myopathy was reported in COVID-19 patients admitted to ICU, indicating a probable correlation between SARS-COV-2 infection and myopathy (Cabañes-Martínez et al. 2020; Nasuelli et al. 2021; Versace et al. 2021).
Myasthenia Gravis
Myasthenia gravis is an autoimmune disorder that causes weakness and rapid fatigue in skeletal muscles due to antibody-mediated blockade of neuromuscular transmission. The first case of post-infectious myasthenia gravis associated with SARS-COV-2 was presented by Huber et al. (2020) in Germany. The patient was a 21-year-old woman developing subacute, vertically shifted double vision evoked by right sided partial oculomotor paresis and ptosis 4 weeks after mild respiratory symptoms. Positive test edrophonium chloride and increased acetylcholine receptor antibodies besides the positive SARS-COV2 antibodies test (IgA/IgG) established the diagnosis of COVID-19 postinfectious myasthenia gravis (Huber et al. 2020). Another study was conducted by Sriwastava et al. (2020) in the USA, in which a 65-year-old woman developed left eyelid ptosis following 2 weeks of fever, cough, and diarrhea. She tested positive for SARS-COV-2 RT-PCR and acetylcholine receptor antibodies (Sriwastava et al. 2020).
Other Complications
Several other neurological complications have been reported in COVID-19 cases with rarity. These manifestations appear to be more bothersome to the patients rather than being fatal. They should not be neglected and infection via SARS-CoV-2 should be suspected in those with such symptoms, despite their scarcity.
Visual changes were reported in several articles. Karadaş et al. (2020) performed a prospective study of 83 COVID-19 patients with neurological manifestations in Turkey. Eight of them developed visual field defects (3.3%) (Karadaş et al. 2020). Selvaraj et al. (2020) reported a 50-year-old man who tested positive for SARS-CoV-2 in the prior week and presented with painless right eye monocular visual disturbance. It was described as a white cloud and blurriness involving most of her right eye visual field, sparing the superior nasal aspect. Her vision recovered spontaneously during the hospitalization (Selvaraj et al. 2020).
Lack of hearing was observed in three patients in Karadaş et al. (2020) study in Turkey. In another study in Turkey, Kilic et al. (2020) evaluated five patients with sole complaint of unilateral sudden sensorineural hearing loss (SSNHL) in SARS-CoV-2 infected patients confirmed by RT-PCR. One of these patients had positive test and responded to hydroxychloroquine, known as COVID-19 treatment according to Republic of Turkey’s Health Ministry COVID-19 guidelines. This demonstrates that non-specific neurological symptoms such as SSNHL could be the only symptom/sign of COVID-19 (Kilic et al. 2020). In China, hearing changes were reported in Lu et al. (2020a, b) article, in which one patient experienced hearing loss (Lu et al. 2020b).
In a study involving 15 Italian hospitals, 43 out of 185 patients (23.2%) developed tinnitus in a period of > 30 to < 60 days after COVID-19 diagnosis with the following characteristics: 17 recurrent (39.5%), 10 occasional (23.3%), seven continuous floating (16.3%), four persistent (9.3%), three pulsatile (7%), and two continuous (4.6%) (Viola et al. 2020). In another study based in Turkey, among 239 patients, five patients (2.1%) had tinnitus, four (1.7%) had restless leg syndrome, and six (2.5%) had balance disorder (Karadaş et al. 2020).
In Iran, Paybast and her colleagues reported a patient with persistent positional vertigo with a history of malaise and sore throat. He was diagnosed with COVID-19 after performing chest CT (Paybast et al. 2020). Vertigo was mentioned as a manifestation of COVID-19 illness in other reports (García-Azorín et al. 2020; Karimi-Galougahi et al. 2020).
A 70-year-old man admitted to a hospital in Spain with a 7-day history of fever, and COVID-19 was diagnosed for him. He developed progressive tremor 17 days after symptom onset followed by gait instability and ataxia. Clonazepam was prescribed and resulted in slight improvement. His symptoms gradually resolved within a month after discharge (Diezma-Martín et al. 2020). Ataxia was also reported in Mao et al. (2020) study in China with a prevalence of one out of 214 patients (Mao et al. 2020). Rábano-Suárez et al. (2020) reported three COVID-19 patients in Spain, who developed generalized myoclonus and improved following immunotherapy (Rábano-Suárez et al. 2020). In a study published by Piscitelli et al. (2020) in Italy, a 39-year-old female developed lower limb tremor and abnormal movements seven days after mild COVID-19 symptoms. She was diagnosed with functional movement disorder (Piscitelli et al. 2020).
In France, Helms et al. (2020) reported corticospinal tract involvement in 89 out of 140 COVID-19 patients referred for ARDS (Helms et al. 2020). In another study in France, Kremer et al. (2020) investigated 37 COVID-19 patients with neurological manifestations. Four had clinical signs of corticospinal tract involvement (Kremer, et al. 2020).
In Spain, Palao et al. (2020) described a case of 29-year-old woman with optic neuritis as the first presentation of demyelinating disease 2–3 weeks after COVID-19 infection diagnosis (Palao et al. 2020).
In a study published by Varatharaj et al. (2020) in the UK, enrolling 153 patients with COVID-19, one patient developed CNS vasculitis (Varatharaj et al. 2020). Another study in Italy presented a case of CNS vasculitis triggered with SARS-CoV-2 infection in a 64-year-old male (Vaschetto et al. 2020).
Kajani et al. (2020) reported a case of neuroleptic malignant syndrome in a COVID-19 febrile middle-aged man who had received haloperidol decanoate injection 3 weeks prior to the hospital admission for altered mental status (Kajani et al. 2020). Memory loss was also reported in association with COVID-19 in several studies (Lu et al. 2020b; Pinna 2020).
Conclusion
SARS-CoV-2 pandemic has led to ever-increasing global casualties, and many unanticipated complications throughout the body including the nervous system. Although these neurological complications will undoubtedly increase the disease burden, they might be the presenting symptoms of COVID-19. Better understanding of these potential complications will help physicians to be more attentive to these potential manifestations and might lead to more timely diagnosis and management.
References
Almazeedi S, Al-Youha S, Jamal MH, Al-Haddad A, Al-Muhaini A et al (2020) Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. E Clin Med 24:100448
Altin F, Cingi C, Uzun T, Bal C (2020) Olfactory and gustatory abnormalities in COVID-19 cases. Eur Arch Otorhinolaryngol 277(10):2775–2781
Anand P, Lau KHV, Chung DY, Virmani D, Cervantes-Arslanian AM et al (2020) Posterior reversible encephalopathy syndrome in patients with coronavirus disease 2019: two cases and a review of the literature. J Stroke Cerebrovasc Dis 29(11):105212
Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR et al (2020) Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. Bmj 369:m1996
Azhideh A (2020) COVID-19 Neurological Manifestations Arash Azhideh. Int Clin Neurosci J
Baig AM, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11(7):995–998
Baig AM, Sanders EC (2020) Potential neuroinvasive pathways of SARS-CoV-2: Deciphering the spectrum of neurological deficit seen in coronavirus disease-2019 (COVID-19). J Med Virol 92(10):1845–1857
Belani P, Schefflein J, Kihira S, Rigney B, Delman BN et al (2020) COVID-19 Is an Independent Risk Factor for Acute Ischemic Stroke. AJNR Am J Neuroradiol 41(8):1361–1364
Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, Rodríguez-Jorge F, Natera-Villalba E et al (2020) Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol 27(9):1738–1741
Benger M, Williams O, Siddiqui J, Sztriha L (2020) Intracerebral haemorrhage and COVID-19: Clinical characteristics from a case series. Brain Behav Immun 88:940–944
Bernard-Valnet R, Pizzarotti B, Anichini A, Demars Y, Russo E et al (2020) Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol 27(9):e43–e44
Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF et al (2020) Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatr 91(8):889–891
Boddington NL, Charlett A, Elgohari S, Walker JL, Mcdonald HI et al (2020) COVID-19 in Great Britain: epidemiological and clinical characteristics of the first few hundred (FF100) cases: a descriptive case series and case control analysis. medRxiv:2020.05.18.20086157
Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB et al (2020) Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv:2020.05.18.20099960
Bulfamante G, Chiumello D, Canevini MP, Priori A, Mazzanti M et al (2020) First ultrastructural autoptic findings of SARS -Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol 86(6):678–679
Cabañes-Martínez L, Villadóniga M, González-Rodríguez L, Araque L, Díaz-Cid A et al (2020) Neuromuscular involvement in COVID-19 critically ill patients. Clin Neurophysiol 131(12):2809–2816
Cariddi LP, Damavandi PT, Carimati F, Banfi P, Clemenzi A et al (2020) Reversible encephalopathy syndrome (PRES) in a COVID-19 patient. J Neurol 267(11):3157–3160
Cavalli Eugenio, Bramanti Alessia, Ciurleo Rosella, Tchorbanov Andrey I, Giordano Antonio et al (2020) Entangling COVID-19 associated thrombosis into a secondary antiphospholipid antibody syndrome: diagnostic and therapeutic perspectives (Review). Int J Mol Med 46(3):903–912
Chachkhiani D, Soliman MY, Barua D, Isakadze M, Villemarette-Pittman, NRt al (2020) Neurological complications in a predominantly African American sample of COVID-19 predict worse outcomes during hospitalization. Clin Neurol Neurosurg 197:106173
Chakraborty U, Chandra A, Ray AK, Biswas P (2020) COVID-19-associated acute transverse myelitis: a rare entity. BMJ Case Rep 13(8)
Chen R, Wang K, Yu J, Howard D, French L, Chen Z, Wen C, Xu Z (2020) The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain
Cheruiyot I, Sehmi P, Ominde B, Bundi P, Mislani M et al (2020) Intracranial hemorrhage in coronavirus disease 2019 (COVID-19) patients. Neurol Sci 1–9
Chougar L, Shor N, Weiss N, Galanaud D, Leclercq D et al (2020) Retrospective observational study of brain MRI findings in patients with acute SARS-CoV-2 infection and neurologic manifestations. Radiology 297(3):e313–e323
Coolen T, Lolli V, Sadeghi N, Rovaï A, Trotta N et al (2020) Early postmortem brain MRI findings in COVID-19 non-survivors. medRxiv:2020.05.04.20090316
D'Ascanio L, Pandolfini M, Cingolani C, Latini G, Gradoni P et al (2020) Olfactory dysfunction in COVID-19 patients: prevalence and prognosis for recovering sense of smell. Otolaryngol Head Neck Surg 194599820943530
D’Silva KM, Wallace ZS (2021) COVID-19 and disease-modifying anti-rheumatic drugs. Curr Rheumatol Rep 23(5):28
DaSilva AF, DosSantos MF (2012) The role of sensory fiber demography in trigeminal and postherpetic neuralgias. J Dent Res 91(1):17–24
Ferreira ACAF, Romão TT, Macedo YS, Pupe C, Nascimento OJ (2020) COVID-19 and herpes zoster co-infection presenting with trigeminal neuropathy. Eur J Neurol
DeBiasi RL, Song X, Delaney M, Bell M, Smith K et al (2020) Severe coronavirus disease-2019 in children and young adults in the Washington, DC, Metropolitan Region. J Pediatr 223:199-203.e1
Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ (2014) Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol 807:75–96
Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L et al (2019) Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 12(1)
Díaz-Pérez C, Ramos C, López-Cruz A, Muñoz Olmedo J, Lázaro González J et al (2020) Acutely altered mental status as the main clinical presentation of multiple strokes in critically ill patients with COVID-19. Neurol Sci 41(10):2681–2684
Diezma-Martín AM, Morales-Casado MI, García-Alvarado N, Vadillo Bermejo A, López-Ariztegui N et al (2020) Tremor and ataxia in COVID-19. Neurologia 35(6):409–410
Disease outbreak news (2019) Pneumonia of unknown cause – China - 2019
Divani AA, Andalib S, Di Napoli M, Lattanzi S, Hussain MS et al (2020) Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J Stroke Cerebrovasc Dis 29(8):104941
Dixon L, Varley J, Gontsarova A, Mallon D, Tona F et al (2020) COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm 7(5)
Dmytriw AA, Phan K, Schirmer C, Settecase F, Heran MKS et al (2020) Ischaemic stroke associated with COVID-19 and racial outcome disparity in North America. J Neurol Neurosurg Psychiatr 91(12):1362–1364
Dorche MS, Huot P, Osherov M, Wen D, Saveriano A et al (2020) Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci 117085
DosSantos MF, Devalle S, Aran V, Capra D, Roque NR et al (2020) Neuromechanisms of SARS-CoV-2: A Review. Front Neuroanat 14:37
Elmashala A, Chopra S, Garg A (2020) The neurologic manifestations of coronavirus disease 2019. J Neurol Res
Eshak N, Abdelnabi M, Ball S, Elgwairi E, Creed K et al (2020) Dysautonomia: an overlooked neurological manifestation in a critically ill COVID-19 patient. Am J Med Sci 360(4):427–429
Fagone P, Ciurleo R, Lombardo SD, Iacobello C, Palermo CI et al (2020) Transcriptional landscape of SARS-CoV-2 infection dismantles pathogenic pathways activated by the virus, proposes unique sex-specific differences and predicts tailored therapeutic strategies. Autoimmun Rev 19(7):102571
Falcone MM, Rong AJ, Salazar H, Redick DW, Falcone S et al (2020) Acute abducens nerve palsy in a patient with the novel coronavirus disease (COVID-19). J Aapos 24(4):216–217
Faucher A, Rey PA, Aguadisch E, Degos B (2020) Isolated post SARS-CoV-2 diplopia. J Neurol 267(11):3128–3129
Finsterer J, Scorza FA (2021) Guillain-Barre syndrome in 220 patients with COVID-19. The Egyptian J Neurol Psychiatr Neurosurg 57(1):55
Forestier G, de Beaurepaire I, Bornet G, Boulouis G (2020) Cytotoxic lesion of the corpus callosum as presenting neuroradiological manifestation of COVID-2019 infection. J Neurol 1–3
Foster KJ, Jauregui E, Tajudeen B, Bishehsari F, Mahdavinia M (2020) Smell loss is a prognostic factor for lower severity of coronavirus disease 2019. Ann Allergy Asthma Immunol 125(4):481–483
García-Azorín D, Trigo J, Talavera B, Martínez-Pías E, Sierra Á et al (2020) Frequency and type of red flags in patients with Covid-19 and headache: a series of 104 hospitalized patients. Headache 60(8):1664–1672
Gaur P, Dixon L, Jones B, Lyall H, Jan W (2020) COVID-19-associated cytotoxic lesions of the corpus callosum. AJNR Am J Neuroradiol 41(10):1905–1907
Ghosh R, Dubey S, Finsterer J, Chatterjee S, Ray BK (2020) SARS-CoV-2-associated acute hemorrhagic, necrotizing encephalitis (AHNE) presenting with cognitive impairment in a 44-year-old woman without comorbidities: a case report. Am J Case Rep 21:e925641
Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M et al (2020) Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis 71(15):889–890
Gil-Rodrigo A, Miró Ò, Piñera P, Burillo-Putze G, Jiménez S et al (2020) Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departments. Emergencias 32(4):233–241
Gogia B, Fang X, Rai P (2020) Intracranial hemorrhage in a patient with COVID-19: possible explanations and considerations. Cureus 12(8):e10159
Gómez-Iglesias P, Porta-Etessam J, Montalvo T, Valls-Carbó A, Gajate V et al (2020) An online observational study of patients with olfactory and gustory alterations secondary to SARS-CoV-2 infection. Front Public Health 8:243
Gu J, Gong E, Zhang B, Zheng J, Gao Z et al (2005) Multiple organ infection and the pathogenesis of SARS. J Exp Med 202(3):415–24
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720
Guo Y, Korteweg C, McNutt MA, Gu J (2008) Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res 133(1):4–12
Hamidianjahromi A, Mowla A (2021) Re: The choice of intravenous thrombolysis for acute ischemic stroke under COVID-19 infection. Clin Neurol Neurosurg 202:106501
Helms J, Kremer S, Merdji H, Schenck M, Severac F et al (2020) Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Critical Care 24(1):491
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271-280.e8
Hogan RE, Grinspan Z, Axeen E, Marquis B, Day BK (2020) COVID-19 in patients with seizures and epilepsy: interpretation of relevant knowledge of presenting signs and symptoms. Epilepsy Curr 20(5):312–315
Hong KS, Lee KH, Chung JH, Shin KC, Choi EY et al (2020) Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J 61(5):431–437
Huber M, Rogozinski S, Puppe W, Framme C, Höglinger G et al (2020) Postinfectious onset of myasthenia gravis in a COVID-19 patient. Front Neurol 11:576153
Iadecola C, Anrather J, Kamel H (2020) Effects of COVID-19 on the nervous system. Cell 183(1):16–27.e1
Iroegbu JD, Ifenatuoha CW, Ijomone OM (2020) Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2. Neurol Sci 41(6):1329–1337
Kajani R, Apramian A, Vega A, Ubhayakar N, Xu P et al (2020) Neuroleptic malignant syndrome in a COVID-19 patient. Brain Behav Immun 88:28–29
Karadaş Ö, Öztürk B, Sonkaya AR (2020) A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol Sci 41(8):1991–1995
Karimi-Galougahi M, Naeini AS, Raad N, Mikaniki N, Ghorbani J (2020) Vertigo and hearing loss during the COVID-19 pandemic - is there an association? Acta Otorhinolaryngol Ital
Khan M, Ibrahim RH, Siddiqi SA, Kerolos Y, Al-Kaylani MM et al (2020) COVID-19 and acute ischemic stroke - a case series from Dubai, UAE. Int J Stroke 15(6):699–700
Khoo A, McLoughlin B, Cheema S, Weil RS, Lambert C et al (2020) Postinfectious brainstem encephalitis associated with SARS-CoV-2. J Neurol Neurosurg Psychiatry 91(9):1013–1014
Kilic O, Kalcioglu MT, Cag Y, Tuysuz O, Pektas E et al (2020) Could sudden sensorineural hearing loss be the sole manifestation of COVID-19? An investigation into SARS-COV-2 in the etiology of sudden sensorineural hearing loss. Int J Infect Dis 97:208–211
Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q et al (2020) Features of anosmia in COVID-19. Med Mal Infect 50(5):436–439
Kremer S, Lersy F, de Sèze J, Ferré JC, Maamar A et al (2020) Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology 297(2):E242-e251
Kucuk A, Cumhur Cure M, Cure E (2020) Can COVID-19 cause myalgia with a completely different mechanism? A hypothesis. Clin Rheumatol 39(7):2103–2104
Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J et al (2020) SARS-CoV-2 productively infects human gut enterocytes. Science 369(6499):50–54
Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD et al (2020a) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277(8):2251–2261
Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P et al (2020b) Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med 288(3):335–344
Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK et al (2016) Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis 213(5):712–22
Li Y, Li M, Wang M, Zhou Y, Chang J et al (2020) Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol
Liang W, Feng Z, Rao S, Xiao C, Xue X et al (2020) Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut 69(6):1141–1143
Liguori C, Pierantozzi M, Spanetta M, Sarmati L, Cesta N et al (2020) Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun 88:11–16
Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC et al (2020) Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol 7(11):2221–2230
Lippi G, Wong J, Henry BM (2020) Myalgia may not be associated with severity of coronavirus disease 2019 (COVID-19). World J Emerg Med 11(3):193–194
Lovell N, Maddocks M, Etkind SN, Taylor K, Carey I et al (2020) Characteristics, symptom management, and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J Pain Symptom Manage 60(1):e77–e81
Lu L, Xiong W, Liu D, Liu J, Yang D et al (2020a) New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia 61(6):e49–e53
Lu Y, Li X, Geng D, Mei N, Wu PY et al (2020b) Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. E Clin Med 25:100484
Majidi S, Fifi JT, Ladner TR, Lara-Reyna J, Yaeger KA et al (2020) Emergent large vessel occlusion stroke during New York City’s COVID-19 outbreak: clinical characteristics and paraclinical findings. Stroke 51(9):2656–2663
Malayala SV, Raza A (2020) A case of COVID-19-induced vestibular neuritis. Cureus 12(6):e8918
Mao L, Jin H, Wang M, Hu Y, Chen S et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China. JAMA Neurol 77(6):683–690
Mehraeen E, Salehi MA, Behnezhad F, Moghaddam HR, SeyedAlinaghi S (2020) Transmission modes of COVID-19: a systematic review. Infect Disord Drug Targets
Mehta S, Bhandari S, Mehta S (2020) Brain autopsies in fatal COVID-19 and postulated pathophysiology: more puzzling than a Rubik’s cube. J Clin Pathol 206967
Meinhardt J, Radke J, Dittmayer C, Mothes R, Franz J et al (2020) Olfactory transmucosal SARS-CoV-2 invasion as port of central nervous system entry in COVID-19 patients. bioRxiv:2020.06.04.135012
Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H et al (2020) Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol 77(11):1–7
Mo P, Xing Y, Xiao Y, Deng L, Zhao Q et al (2020) Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis
Mondal R, Lahiri D, Deb S, Bandyopadhyay D, Shome G et al (2020) COVID-19: Are we dealing with a multisystem vasculopathy in disguise of a viral infection? J Thromb Thrombolysis
Moreau A, Ego A, Vandergheynst F, Taccone FS, Sadeghi N et al (2020) Cytotoxic lesions of the corpus callosum (CLOCCs) associated with SARS-CoV-2 infection. J Neurol 1–3
Moriguchi T, Harii N, Goto J, Harada D, Sugawara H et al (2020) A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 94:55–58
Mowla A (2020c) Stroke Care during the COVID-19 Pandemic; A Global Challenge. Iran J Med Sci 45(5):323
Mowla A, Shakibajahromi B, Shahjouei S, Borhani-Haghighi A, Rahimian N et al (2020a) Cerebral venous sinus thrombosis associated with SARS-CoV-2; a multinational case series. J Neurol Sci 419:117183
Mowla A, Sizdahkhani S, Sharifian-Dorche M, Selvan P, Emanuel BA et al (2020b) Unusual pattern of arterial macrothrombosis causing stroke in a young adult recovered from COVID-19. J Stroke Cerebrovasc Dis 29(12):105353
Munz M, Wessendorf S, Koretsis G, Tewald F, Baegi R et al (2020) Acute transverse myelitis after COVID-19 pneumonia. J Neurol 267(8):2196–2197
Mutiawati E, Syahrul S, Fahriani M, Fajar JK, Mamada SS et al (2020) Global prevalence and pathogenesis of headache in COVID-19: A systematic review and meta-analysis [version 1; peer review: 1 approved with reservations]. F1000 Res 9(1316)
Nasuelli NA, Pettinaroli R, Godi L, Savoini C, De Marchi F et al (2021) Critical illness neuro-myopathy (CINM) and focal amyotrophy in intensive care unit (ICU) patients with SARS-CoV-2: a case series. Neurol Sci 42(3):1119–1121
Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82(15):7264–75
Neto BD, Fornazieri MA, Dib C, Di Francesco RC, Doty RL et al (2020) Chemosensory dysfunction in COVID-19: prevalences, recovery rates, and clinical associations on a large Brazilian sample. Otolaryngol Head Neck Surg 194599820954825
Ortiz-Brizuela E, Villanueva-Reza M, González-Lara MF, Tamez-Torres KM, Román-Montes CM et al (2020) Clinical and epidemiological characteristics of patients diagnosed with COVID-19 in a tertiary care center in Mexico City: a prospective cohort study. Rev Invest Clin 72(3):165–177
Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H et al (2020) Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 382(20):e60
Paderno A, Mattavelli D, Rampinelli V, Grammatica A, Raffetti E et al (2020) Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Head Neck Surg 163(6):1144–1149
Palao M, Fernández-Díaz E, Gracia-Gil J, Romero-Sánchez CM, Díaz-Maroto I et al (2020) Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord 45:102377
Paniz-Mondolfi Alberto, Bryce Clare, Grimes Zachary, Gordon Ronald E, Reidy Jason et al (2020) Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Journal of Medical Virology 92(7):699–702
Parsons T, Banks S, Bae C, Gelber J, Alahmadi H et al (2020) COVID-19-associated acute disseminated encephalomyelitis (ADEM). J Neurol 267(10):2799–2802
Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S et al (2020) The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 143(10):3104–3120
Paybast S, Emami A, Koosha M, Baghalha F (2020) Novel coronavirus disease (COVID-19) and central nervous system complications: what neurologist need to know. Acta Neurol Taiwan 29(1):24–31
Pezzini A, Padovani A (2020) Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol 16(11):636–644
Pfefferkorn T, Dabitz R, von Wernitz-Keibel T, Aufenanger J, Nowak-Machen M et al (2020) Acute polyradiculoneuritis with locked-in syndrome in a patient with Covid-19. J Neurol 267(7):1883–1884
Pinna P, Grewal P, Hall JP, Tavarez T, Dafer RM et al (2020) Neurological manifestations and COVID-19: Experiences from a tertiary care center at the Frontline. J Neurol Sci 415:116969
Pinna P (2020) Neurological manifestations and COVID-19: experiences from a tertiary care center at the Frontline. J Neurol Sci
Piscitelli D, Perin C, Tremolizzo L, Peroni F, Cerri CG et al (2020) Functional movement disorders in a patient with COVID-19. Neurol Sci 41(9):2343–2344
Poyiadji N, Cormier P, Patel PY, Hadied MO, Bhargava P et al (2020) Acute Pulmonary Embolism and COVID-19. Radiology 201955
Pranata R, Huang I, Lim MA, Wahjoepramono J, July J (2020) Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis 29(8):104949
Rábano-Suárez P, Bermejo-Guerrero L, Méndez-Guerrero A, Parra-Serrano J, Toledo-Alfocea D et al (2020) Generalized myoclonus in COVID-19. Neurology 95(6):e767–e772
Reichard R. Ross, Kashani Kianoush B, Boire Nicholas A, Constantopoulos E, Guo Yong et al (2020) Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathologica 140(1):1–6
Remmelink M, De Mendonça R, D’Haene N, De Clercq S, Verocq C et al (2020) Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Critical Care 24(1):495
Rep MMWR, Morb Mortal Wkly (2020) Coronavirus disease 2019 in children - United States. 69(14):422–426
Richardson PJ, Ottaviani S, Prelle A, Stebbing J, Casalini G et al (2020) CNS penetration of potential anti-COVID-19 drugs. J Neurol 267(7):1880–1882
Riva N, Russo T, Falzone YM, Strollo M, Amadio S et al (2020) Post-infectious Guillain-Barré syndrome related to SARS-CoV-2 infection: a case report. J Neurol 267(9):2492–2494
Salepci E, Turk B, Ozcan SN, Bektas ME, Aybal A et al (2020) Symptomatology of COVID-19 from the otorhinolaryngology perspective: a survey of 223 SARS-CoV-2 RNA-positive patients. Eur Arch Otorhinolaryngol 1–11
Savić D, Alsheikh TM, Alhaj AK, Lazovic L, Alsarraf L et al (2020) Ruptured cerebral pseudoaneurysm in an adolescent as an early onset of COVID-19 infection: case report. Acta Neurochir (Wien) 162(11):2725–2729
Scoppettuolo P, Serena B, Gilles N (2020) Neurological involvement in SARS-CoV-2 infection: a clinical systematic review. Brain Behav Immun Health 5:100094
Scullen T, Keen J, Mathkour M, Dumont AS, Kahn L (2020) Coronavirus 2019 (COVID-19)-associated encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg 141:e437–e446
Selvaraj V, Sacchetti D, Finn A, Dapaah-Afriyie K (2020) Acute vision loss in a patient with COVID-19. R I Med J 103(6):37–38
Shah VA, Nalleballe K, Zaghlouleh ME, Onteddu S (2020) Acute encephalopathy is associated with worse outcomes in COVID-19 patients. Brain Behav Immun Health 8:100136
Shahjouei S, Anyaehie M, Koza E, Tsivgoulis G, Naderi S et al (2021) SARS-CoV-2 is a culprit for some, but not all acute ischemic strokes: a report from the Multinational COVID-19 Stroke Study Group. J Clin Med 10(5):931
Shahjouei S, Naderi S, Li J, Khan A, Chaudhary D et al (2020) Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. E Bio Med 59:102939
Shakibajahromi B, Borhani-Haghighi A, Haseli S, Mowla A (2020a) Cerebral venous sinus thrombosis might be under-diagnosed in the COVID-19 era. Eneurologicalsci 20:100256
Shakibajahromi B, Borhani-Haghighi A, Haseli S, Mowla A (2020b) Cerebral venous sinus thrombosis might be under-diagnosed in the COVID-19 era. Eneurologicalsci 20
Sharifi-Razavi A, Karimi N, Rouhani N (2020) COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect 35:100669
Sharifian-Dorche M, Mowla A (2020) Neurological care during the pandemic; a global challenge. J Exp Clin Neurosci 7(2)
Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K et al (2020) Neuropathological features of Covid-19. New Eng J Med 383(10):989–992
Sotoca J, Rodríguez-Álvarez Y (2020) COVID-19-associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflamm 7(5)
Spadera L, Viola P, Pisani D, Scarpa A, Malanga D et al (2020) Sudden olfactory loss as an early marker of COVID-19: a nationwide Italian survey. Eur Arch Otorhinolaryngol 1–9
Sriwastava S, Tandon M, Kataria S, Daimee M, Sultan S (2020) New onset of ocular myasthenia gravis in a patient with COVID-19: a novel case report and literature review. J Neurol 1–7
Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H et al (2020) Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in Metropolitan Detroit. JAMA Netw Open 3(6):e2012270
Sungnak W, Huang N, Bécavin C, Berg M, Queen R et al (2020) SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26(5):681–687
Sweid A, Hammoud B, Bekelis K, Missios S, Tjoumakaris SI et al (2020) Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke 15(7):733–742
Tancheva L Petralia MC, Miteva S, Dragomanova S, Solak A et al (2020) Emerging neurological and psychobiological aspects of COVID-19 infection. Brain Sci 10(11):852
Taylor BES, Khandelwal P, Rallo MS, Patel P, Smith L et al (2020) Outcomes and spectrum of major neurovascular events among COVID-19 patients: a 3-center experience. Neurosurg Open 1(3):okaa008
Toptan T, Aktan Ç, Başarı A, Bolay H (2020) Case series of headache characteristics in COVID-19: headache can be an isolated symptom. Headache 60(8):1788–1792
Trigo J, García-Azorín D, Planchuelo-Gómez Á, Martínez-Pías E, Talavera B et al (2020) Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: a retrospective cohort study. J Headache Pain 21(1):94
Trojanowicz B, Ulrich C, Kohler F, Bode V, Seibert E et al (2017) Monocytic angiotensin-converting enzyme 2 relates to atherosclerosis in patients with chronic kidney disease. Nephrol Dial Transplant 32(2):287–298
TunÇ A, ÜnlÜbaŞ Y, Alemdar M, AkyÜz E (2020) Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci 77:227–229
Usman AA, Han J, Acker A, Olia SE, Bermudez C et al (2020) A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID-19. J Cardiothorac Vasc Anesth 34(11):3006–3012
Uygun Ö, Ertaş M, Ekizoğlu E, Bolay H, Özge A et al (2020) Headache characteristics in COVID-19 pandemic-a survey study. J Headache Pain 21(1):121
Vacchiano V, Riguzzi P, Volpi L, Tappatà M, Avoni P et al (2020) Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci 41(8):2029–2031
Vahabizad F, Dorche MS, Mohammadi P, Khatibi K, Mowla A (2020) COVID-19-related acute ischemic stroke in young adults: what is the optimal antithrombotic regimen for secondary prevention? J Neurol Res 10(5):203–206
Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A et al (2020) Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 42(7):1560–1569
Valiuddin H, Skwirsk B, Paz-Arabo P (2020) Acute transverse myelitis associated with SARS-CoV-2: A Case-Report. Brain Behav Immun Health 5:100091
Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA et al (2020) Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 7(10):875–882
Vaschetto R, Cena T, Sainaghi PP, Meneghetti G, Bazzano S et al (2020) Cerebral nervous system vasculitis in a Covid-19 patient with pneumonia. J Clin Neurosci 79:71–73
Versace V, Sebastianelli L, Ferrazzoli D, Saltuari L, Kofler M et al (2021) Case report: Myopathy in critically ill COVID-19 patients: a consequence of hyperinflammation? Front Neurol 12:625144
Viola P, Ralli M, Pisani D, Malanga D, Sculco D et al (2020) Tinnitus and equilibrium disorders in COVID-19 patients: preliminary results. Eur Arch Oto-Rhino-Laryngol 1-6
Vohora D, Jain S, Tripathi M, Potschka H (2020) COVID-19 and seizures: Is there a link? Epilepsia 61(9):1840–1853
von Weyhern CH, Kaufmann I, Neff F, Kremer M (2020) Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet 395(10241):e109
Vonck K, Garrez I, De Herdt V, Hemelsoet D, Laureys G et al (2020) Neurological manifestations and neuro-invasive mechanisms of the severe acute respiratory syndrome coronavirus type 2. Eur J Neurol 27(8):1578–1587
Wang D, Yin Y, Hu C, Liu X, Zhang X et al (2020) Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care 24(1):188
Wang H, Yang P, Liu K, Guo F, Zhang Y et al (2008) SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res 18(2):290–301
World Health Organization (2019) Novel Coronavirus (2019-nCoV) situation report-1
World Health Organization (2020) Coronavirus Disease (COVID-19) Dashboard
Wu Y, Xu X, Chen Z, Duan J, Hashimoto K et al (2020) Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 87:18–22
Wuhan Municipal Health Commission (2020) Report of clustering pneumonia of unknown etiology in Wuhan City
Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E et al (2020) SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke 51(7):2002–2011
Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS (2020) Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol 10(7):806–813
Yavarpour-Bali H, Ghasemi-Kasman M (2020) Update on neurological manifestations of COVID-19. Life Sci 257:118063
Ye M, Ren Y, Lv T (2020) Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun 88:945–946
Yonker LM, Neilan AM, Bartsch Y, Patel AB, Regan J et al (2020) Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J Pediatr 227:45-52.e5
Zayet S, Klopfenstein T, Mercier J, Kadiane-Oussou NJ, Lan Cheong Wah L et al (2020) Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection 1–5
Zhang G, Hu C, Luo L, Fang F, Chen Y et al (2020) Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol 127:104364
Zhao H, Shen D, Zhou H, Liu J, Chen S (2020) Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 19(5):383–384
Zhou J, Li C, Liu X, Chiu MC, Zhao X et al (2020) Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med 26(7):1077–1083
Zou L, Ruan F, Huang M, Liang L, Huang H et al (2020) SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382(12):1177–1179
Acknowledgements
Part of the figures in this article was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; http://smart.servier.com.
Author information
Authors and Affiliations
Contributions
HJ and MJ performed the literature search and data analysis, drafted, and revised the work. AAH supervised the process and drafted and revised the work. MM, AM, and HP critically revised the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jafari Khaljiri, H., Jamalkhah, M., Amini Harandi, A. et al. Comprehensive Review on Neuro-COVID-19 Pathophysiology and Clinical Consequences. Neurotox Res 39, 1613–1629 (2021). https://doi.org/10.1007/s12640-021-00389-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00389-z