Abstract
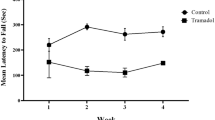
Tramadol is a synthetic analogue of codeine that is often prescribed for the treatment of mild to moderate pains. It has a number of side effects including emotional instability and anxiety. In this study, we focus on the structural and functional changes of prefrontal cortex under chronic exposure to tramadol. At the cellular level, the amounts of ROS and annexin V in PC12 cells were evidently increased upon exposure to tramadol (at a concentration of 600 μM for 48 h). To this end, the rats were daily treated with tramadol at doses of 50 mg/kg for 3 weeks. Our findings reveal that tramadol provokes atrophy and apoptosis by the induction of apoptotic markers such as Caspase 3 and 8, pro-inflammatory markers, and downregulation of GDNF. Moreover, it triggers microgliosis and astrogliosis along with neuronal death in the prefrontal cortex. Behavioral disturbance and cognitive impairment are other side effects of tramadol. Overall, our results indicate tramadol-induced neurodegeneration in the prefrontal cortex mainly through activation of neuroinflammatory response.








Similar content being viewed by others
References
Afshari R, Ghooshkhanehee H (2009) Tramadol overdose induced seizure, dramatic rise of CPK and acute renal failure JPMA. J Pak Med Assoc 59:178
Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394
Aktas O, Ullrich O, Infante-Duarte C, Nitsch R, Zipp F (2007) Neuronal damage in brain inflammation. Arch Neurol 64:185–189
Babalonis S, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL (2013) Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug Alcohol Depend 129:116–124
Baghishani F, Mohammadipour A, Hosseinzadeh H, Hosseini M, Ebrahimzadeh-Bideskan A (2018) The effects of tramadol administration on hippocampal cell apoptosis, learning and memory in adult rats and neuroprotective effects of crocin. Metab Brain Dis 33:907–916
Barsotti CE, Mycyk MB, Reyes J (2003) Withdrawal syndrome from tramadol hydrochloride. Am J Emerg Med 21:87–88
Beakley BD, Kaye AM, Kaye AD (2015) Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician 18:395–400
Boroujeni ME, Gardaneh M, Shahriari MH, Aliaghaei A, Hasani S (2017) Synergy between choroid plexus epithelial cells-conditioned medium and knockout serum replacement converts human adipose-derived stem cells to dopamine-secreting neurons. Rejuvenation Res 20(4):309–319
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436:193–200
Caspani O, Reitz M-C, Ceci A, Kremer A, Treede R-D (2014) Tramadol reduces anxiety-related and depression-associated behaviors presumably induced by pain in the chronic constriction injury model of neuropathic pain in rats. Pharmacol Biochem Behav 124:290–296
Chapman BS, Kuntz ID (1995) Modeled structure of the 75-kDa neurotrophin receptor. Protein Sci 4:1696–1707
Chen C, Li X, Ge G, Liu J, Biju KC, Laing SD, Qian Y, Ballard C, He Z, Masliah E, Clark RA, O’Connor JC, Li S (2018) GDNF-expressing macrophages mitigate loss of dopamine neurons and improve Parkinsonian symptoms in MitoPark mice. Sci Rep 8:5460
Corder KM, Cortes MA, Bartley AF, Lear SA, Lubin FD, Dobrunz LE (2018) Prefrontal cortex-dependent innate behaviors are altered by selective knockdown of Gad1 in neuropeptide Y interneurons. PLoS One 13(7)
Dheen ST, Kaur C, Ling EA (2007) Microglial activation and its implications in the brain diseases. Curr Med Chem 14:1189–1197. https://doi.org/10.2174/092986707780597961
El-Baky AE, Hafez MM (2017) NOS expression in oxidative stress, neurodegeneration and male infertility induced by the abuse of tramadol. Biochem Pharmacol 6:1–6
Eskandarian Boroujeni M, Peirouvi T, Shaerzadeh F, Ahmadiani A, Abdollahifar MA et al (2019) Differential gene expression and stereological analyses of the cerebellum following methamphetamine exposure. Addict Biol 25:e12707
Ferrara N (2002) Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol 29:10–14
Ghoneim FM, Khalaf HA, Elsamanoudy AZ, Helaly AN (2014) Effect of chronic usage of tramadol on motor cerebral cortex and testicular tissues of adult male albino rats and the effect of its withdrawal: histological, immunohistochemical and biochemical study. Int J Clin Exp Pathol 7:7323–7341
Golsorkhdan SA, Boroujeni ME, Aliaghaei A, Abdollahifar MA, Ramezanpour A et al (2019) Methamphetamine administration impairs behavior, memory and underlying signaling pathways in the hippocampus. Behav Brain Res:112300
Goodman JC, Van M, Gopinath SP, Robertson CS (2008) Pro-inflammatory and pro-apoptotic elements of the neuroinflammatory response are activated in traumatic brain injury. Acta Neurochir Suppl 102:437–439
Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci 73:2424–2428
Guadagno J (2015) Mechanisms of neural precursor cell apoptosis by microglia-derived cytokines. Mediat Inflamm 4:5–15
Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A et al (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394
Haanen C, Vermes I (1995) Apoptosis and inflammation. Mediat Inflamm 4:5–15
Hashimoto M, Nitta A, Fukumitsu H, Nomoto H, Shen L, Furukawa S (2005) Involvement of glial cell line-derived neurotrophic factor in activation processes of rodent macrophages. J Neurosci Res 79:476–487
Hawton K, Bergen H, Simkin S, Wells C, Kapur N et al (2012) Six-year follow-up of impact of co-proxamol withdrawal in England and Wales on prescribing and deaths: time-series study. PLoS Med 9:1001213–1001213
He Y, Taylor N, Fourgeaud L, Bhattacharya A (2017) The role of microglial P2X7: modulation of cell death and cytokine release. J Neuroinflammation 14:135–135
Hennies HH, Friderichs E, Schneider J (1988) Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung 38:877–880
Honda S, Nakajima K, Nakamura Y, Imai Y, Kohsaka S (1999) Rat primary cultured microglia express glial cell line-derived neurotrophic factor receptors. Neurosci Lett 275:203–206
Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D (2015) Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation 12:114
Huang Z, Zhou T, Sun X, Zheng Y, Cheng B, Li M, Liu X, He C (2018) Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ 25:180–189
Kahn LH, Alderfer RJ, Graham DJ (1997) Seizures reported with tramadol. JAMA 278:1661
Khedr EM, Gabra RH, Noaman M, Abo Elfetoh N, Farghaly HSM (2016) Cortical excitability in tramadol dependent patients: a transcranial magnetic stimulation study. Drug Alcohol Depend 169:110–116
Kruidering M, Evan GI (2000) Caspase-8 in apoptosis: the beginning of “the end”? IUBMB Life 50:85–90
Lagard C, Chevillard L, Malissin I, Risede P, Callebert J et al (2016) Mechanisms of tramadol-related neurotoxicity in the rat: does diazepam/tramadol combination play a worsening role in overdose? Toxicol Appl Pharmacol 310:108–119
Liu JT, Dong MH, Zhang JQ, Bai Y, Kuang F et al (2015) Microglia and astroglia: the role of neuroinflammation in lead toxicity and neuronal injury in the brain neuroimmunology and neuroinflammation. Neuroimmu Neuroinflam 2:131–137
Mintzer MZ, Lanier RK, Lofwall MR, Bigelow GE, Strain EC (2010) Effects of repeated tramadol and morphine administration on psychomotor and cognitive performance in opioid-dependent volunteers. Drug Alcohol Depend 111:265–268
Mwangi SM, Li G, Ye L, Liu Y, Reichardt F, Yeligar SM, Hart CM, Czaja MJ, Srinivasan S (2019) Glial cell line-derived Neurotrophic factor enhances autophagic flux in mouse and rat hepatocytes and protects against palmitate lipotoxicity. Hepatology 69:2455–2470
Nafea OE, ElKhishin IA, Awad OA, Mohamed DA (2016) A study of the neurotoxic effects of tramadol and cannabis in adolescent male albino rats. Int J Sci Rep 2(7):143–154
Pocock JM, Liddle AC (2001) Microglial signalling cascades in neurodegenerative disease. Prog Brain Res 132:555–565
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
Preston KL, Jasinski DR, Testa M (1991) Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend 27:7–17
Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE et al (1992) Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 260:275–285
Ragab IK, Mohamed HZE (2017) Histological changes of the adult albino rat’s entorhinal cortex under the effect of tramadol administration: histological and morphometric study. Alexandria J Med 53:123–133
Rajabizadeh G, Kheradmand A, Nasirian M (2009) Psychosis following tramadol withdrawal. Addict Health 1(1):58
Ramesh G, MacLean AG, Philipp MT (2013) Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediat Inflamm 2013:480739
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 361:1545–1564
Rocha SM, Cristovao AC, Campos FL, Fonseca CP, Baltazar G (2012) Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiol Dis 47:407–415
Rock KL, Kono H (2008) The inflammatory response to cell death. Annu Rev Pathol 3:99–126
Schratzberger P, Schratzberger G, Silver M, Curry C, Kearney M, Magner M, Alroy J, Adelman LS, Weinberg DH, Ropper AH, Isner JM (2000) Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat Med 6:405–413
Skaper SD (2008) The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neuro Dis Drug Targets 7:46–62
Slotkin TA (2005) Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC (ed) Toxicity of organophosphate and carbamate pesticides. Elsevier Academic Press, San Diego, pp 293–314
Spiller HA, Gorman SE, Villalobos D, Benson BE, Ruskosky DR, Stancavage MM, Anderson DL (1997) Prospective multicenter evaluation of tramadol exposure. J Toxicol Clin Toxicol 35:361–364
Streit WJ, Mrak RE, Griffin WST (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 1:14–14
Symeon I, Polissidis A, Balafas E, Stasinopoulou M, Alexakos P, Voyiatzaki C, Kostomitsopoulos N (2017) Evaluation of the effects of tramadol on analgesic response and locomotor activity on two different strains of laboratory mice. J Hellenic Vet Med Soc 68(1):089–096
Szkutnik-Fiedler D, Kus K, Balcerkiewicz M, Grześkowiak E, Nowakowska E, Burda K, Ratajczak P, Sadowski C (2012) Concomitant use of tramadol and venlafaxine- evaluation of antidepressant-like activity and other behavioral effects in rats. Pharmacol Rep 64:1350–1358
Tehrani AM, Boroujeni ME, Aliaghaei A, Feizi MAH et al (2019) Methamphetamine induces neurotoxicity-associated pathways and stereological changes in prefrontal cortex. Neurosci Lett:134478
Tzschentke TM, Bruckmann W, Friderichs E (2002) Lack of sensitization during place conditioning in rats is consistent with the low abuse potential of tramadol. Neurosci Lett 329:25–28
Venero JL, Burguillos MA, Joseph B (2013) Caspases playing in the field of neuroinflammation: old and new players developmental. Neuroscience 35:88–101
Yang Y, Jiang G, Zhang P, Fan J (2015) Programmed cell death and its role in inflammation. Mil Med Res 2:12–12
Yeiser EC, Rutkoski NJ, Naito A, Inoue J, Carter BD (2004) Neurotrophin signaling through the p75 receptor is deficient in traf6−/− mice. J Neurosci 24:10521–10529
Acknowledgments
This article has been extracted from the MSc thesis written by F. Aghajanpour and the present article is financially supported by School of Medicine, Shahid Beheshti University of Medical Sciences (Registration No: 16116).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The research was approved by the Animal Care and Use Committee of Shahid Beheshti University of Medical Sciences (IR SBMU.MSP.REC.1398.292).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aghajanpour, F., Boroujeni, M.E., Jahanian, A. et al. Tramadol: a Potential Neurotoxic Agent Affecting Prefrontal Cortices in Adult Male Rats and PC-12 Cell Line. Neurotox Res 38, 385–397 (2020). https://doi.org/10.1007/s12640-020-00214-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00214-z