Abstract
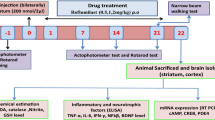
Huntington disease is hyperkinetic movement disorder characterized by selective and immense degradation of GABAergic medium spiny neurons in striatum. Quinolinic acid (QA)-induced neurotoxicity involves a cascade of events such as excitotoxicity, ATP depletion, oxidative stress, neuroinflammation, as well as selective GABAergic neuronal loss. Therefore, we investigated spermidine, an endogenous molecule with free radical scavenging, anti-inflammatory, and N-methyl-d-aspartate receptor antagonistic properties, for its beneficial potential if any, in QA-induced Huntington’s like symptoms in rats. Rats were administered with QA (200 nmol/2 µl saline) bilaterally on 0 day. Spermidine (5 and 10 mg/kg, p.o.) was administered for 21 days once a day. Behavioral parameters (body weight, locomotor activity, grip strength, and narrow beam walk) observations were done on 1st, 7th, 14th, and 21st day after QA treatment. On 21st day, animals were sacrificed and rat striatum was isolated for biochemical (LPO, GSH, Nitrite), neuroinflammation (TNF-α, IL-1β, and IL-6), and neurochemical analysis (GABA, glutamate, dopamine, norepinephrine, serotonin, DOPAC, HVA, 5-HIAA, adenosine, adenine, hypoxanthine, and inosine). QA treatment significantly altered body weight, locomotor activity, motor coordination, oxidative defense (increased LPO, nitrite, and decreased GSH), pro-inflammatory levels (TNF-α, IL-6 and IL-1β), GABA, glutamate, catecholamines level (norepinephrine, dopamine, and serotonin and their metabolites), and purines level (adenosine, inosine, and hypoxanthine). Spermidine (5 and 10 mg/kg, p.o.) significantly attenuated these alterations in body weight, motor impairments, oxidative stress, neuroinflammatory markers, GABA, glutamate, catecholamines, adenosine, and their metabolites levels in striatum. The neuroprotective effect of spermidine against QA-induced excitotoxic cell death is attributed to its antioxidant, N-methyl-d-aspartate receptor antagonistic, anti-inflammatory properties, and prevention of neurotransmitters alteration in striatum.











Similar content being viewed by others
Abbreviations
- QA:
-
Quinolinic acid
- HD:
-
Huntington’s disease
- LPO:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- MSNs:
-
Medium spiny neurons
- NF-кB:
-
Nuclear factor-kappa beta
- NMDA:
-
N-methyl-d-aspartate
- ROS:
-
Reactive oxygen species
- TNF-α:
-
Tumor necrosis factor-alpha
- IL-1β:
-
Interleukin-1 βeta
- DOPAC:
-
3,4-Dihydroxyphenylacetic acid
- HVA:
-
Homovanillic acid
- 5-HIAA:
-
5-Hydroxy 3-indole acetic acid
References
Akula KK, Kaur M, Bishnoi M, Kulkarni SK (2008) Development and validation of an RP-HPLC method for the estimation of adenosine and related purines in brain tissues of rats. J Sep Sci 31(18):3139–3147
Aldinio C, Mazzari S, Toffano G, Köhler C, Schwarcz R (1985) Effects of intracerebral injections of quinolinic acid on serotonergic neurons in the rat brain. Brain Res 341(1):57–65
Beal MF (1992) Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol 31:119–130
Beal MF (1994) Neurochemistry and toxin models in Huntington’s disease. Curr Opin Neurol 7:542–547
Belle NAV, Dalmolin GD, Fonini G, Rubin MA, Rocha JBT (2004) Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res 1008(2):245–251
Belle NA, Dalmolin GD, Fonini G, Gindri Sinhorin VD, Ferreira da Silveira A, Rubin MA et al (2008) Spermine attenuates behavioral and biochemical alterations induced by quinolinic acid in the striatum of rats. Brain Res 1198:107–114
Blum D, Gall D, Galas MC, d’Alcantara P, Bantubungi K, Schiffmann SN (2002) The adenosine A1 receptor agonist adenosine amine congener exerts a neuroprotective effect against the development of striatal lesions and motor impairments in the 3-nitropropionic acid model of neurotoxicity. J Neurosci 22:9122–9133
Blum D, Hourez R, Galas MC, Popoli P, Schiffmann SN (2003) Adenosine receptors in Huntington’s disease. Lancet Neurol 2:366–374
Brouillet E (2014) The 3-NP model of striatal neurodegeneration. Curr Protoc Neurosci. 9(48):1–9
Brown P, Dale N (2000) Adenosine A1 receptors modulate high voltage-activated Ca2+ currents and motor pattern generation in the Xenopus embryo. J Physiol 525(3):655–667
Caraceni T, Girotti F, Giovannini P, Pederzoli M, Parati E (1979) Effects of DA agonist in Huntington disease hyperkinesia. Ital J Neurol Sci 1(2):155–161
Carter C (1994) Neuropharmacology of Polyamines. Academic Press, Londres
Carvalho FB, Mello CF, Marisco PC, Tonello R, Girardi BA, Ferreira J et al (2012) Spermidine decreases Na+, K+-ATPase activity through NMDA receptor and protein kinase G activation in the hippocampus of rats. Eur J Pharmacol 684(1):79–86
Charara A, Heilman C, Levey A, Smith Y (1999) Pre-and postsynaptic localization of GABA B receptors in the basal ganglia in monkeys. Neuroscience 95(1):127–140
Chen JY, Wang EA, Cepeda C, Levine MS (2013) Dopamine imbalance in Huntington’s disease: a mechanism for the lack of behavioral flexibility. Front Neurosci. 7:114
Coert BA, Anderson RE, Meyer FB (2000) Exogenous spermine reduces ischemic damage in a model of focal cerebral ischemia in the rat. Neurosci Lett 282(1):5–8
Dale N, Gilday D (1996) Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos. Nature 383(6597):259–263
Donzanti BA, Yamamoto BK (1988) An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci 43:913–922
Drew PD, Chavis JA (2000) Inhibition of microglial cell activation by cortisol. Brain Res Bull 52(5):391–396
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Gallagher MJ, Huang H, Grant ER, Lynch DR (1997) The NR2B-specific interactions of polyamines and protons with the N-methyl-d-aspartate receptor. J Biol Chem 272(40):24971–24979
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem 126(1):131–138
Jhamandas K, Boegman R, Beninger R, Miranda A, Lipic K (2000) Excitotoxicity of quinolinic acid: modulation by endogenous antagonists. Neurotox Res 2(2–3):139–155
Jongen P, Renier W, Gabreels F (1980) Seven cases of Huntington’s disease in childhood and levodopa induced improvement in the hypokinetic—rigid form. Clin Neurol Neurosurg 82(4):251–261
Kalonia H, Mishra J, Kumar A (2012) Targeting neuro-inflammatory cytokines and oxidative stress by minocycline attenuates quinolinic-acid-induced Huntington’s disease-like symptoms in rats. Neurotox Res 22(4):310–320
Khan A, Jamwal S, Bijjem KRV, Prakash A, Kumar P (2015) Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3β modulators in 3-nitropropionic acid induced neurotoxicity in rats. Neuroscience 287:66–77
Kumar P, Kalonia H, Kumar A (2010) Nitric oxide mechanism in the protective effect of antidepressants against 3-nitropropionic acid induced cognitive deficit, glutathione and mitochondrial alteration in animal model of Huntington’s disease. Behav Pharmacol 21(3):217–230
Kumar P, Kalonia H, Kumar A (2011) Novel protective mechanisms of antidepressants against 3-nitroproprionic acid induced Huntington’s-like symptoms: a comparative study. J Psychopharmacol 25(10):1399–1411
Kumar P, Kalonia H, Kumar A (2012) Possible GABAergic mechanism in the neuroprotective effect of gabapentin and lamotrigine against 3-nitropropionic acid induced neurotoxicity. Eur J Pharmacol 674(2):265–274
Kumar P, Kumar P, Khan A, Deshmukh R, Lal Sharma P (2014) Role of neurosteroids in experimental 3-nitropropionic acid induced neurotoxicity in rats. Eur J Pharmacol 723:38–45
Lacey C, Boyes J, Gerlach O, Chen L, Magill P, Bolam J (2005) GABA B receptors at glutamatergic synapses in the rat striatum. Neuroscience 136(4):1083–1095
Leegwater-Kim J, Cha J-HJ (2004) The paradigm of Huntington’s disease: therapeutic opportunities in neurodegeneration. NeuroRx. 1(1):128–138
Lewandowski NM, Ju S, Verbitsky M, Ross B, Geddie ML, Rockenstein E et al (2010) Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proc Natl Acad Sci 107(39):16970–16975
Lovaas E (1996) Antioxidative and metal-chelating effects of polyaminesI. Antioxid Dis Mech Ther Strateg 38:119
Lovaas E, Carlin G (1991) Spermine: an anti-oxidant and anti-inflammatory agent. Free Radic Biol Med 11(5):455–461
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Madeo F, Eisenberg T, Büttner S, Ruckenstuhl C, Kroemer G (2010) Spermidine: a novel autophagy inducer and longevity elixir. Autophagy. 6(1):160–162
Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma P (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698(1):6–18
Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD et al (2010) Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron 65(2):178–190
Minois N, Carmona-Gutierrez D, Madeo F (2011) Polyamines in aging and disease. Aging (Albany NY). 3(8):716
Mishra J, Kumar A (2014) Improvement of mitochondrial function by paliperidone attenuates quinolinic acid-induced behavioural and neurochemical alterations in rats: implications in Huntington’s disease. Neurotox Res 26(4):363–381
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic press, San Diego
Pérez-De La Cruz V, Carrillo-Mora P, Santamaría A (2012) Quinolinic acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. IJTR. 5:1
Ramaswamy S, McBride JL, Kordower JH (2007) Animal models of Huntington’s disease. ILAR J 48(4):356–373
Rock DM, Macdonald RL (1995) Polyamine regulation of N-methyl-d-aspartate receptor channels. Annu Rev Pharmacol Toxicol 35(1):463–482
Standaert DG, Friberg IK, Landwehrmeyer GB, Young AB, Penney J (1999) Expression of NMDA glutamate receptor subunit mRNAs in neurochemically identified projection and interneurons in the striatum of the rat. Mol Brain Res 64(1):11–23
Svenningsson P, Le Moine C, Fisone G, Fredholm BB (1999) Distribution, biochemistry and function of striatal adenosine A 2A receptors. Prog Neurobiol 59:355–396
Thakur P, Nehru B (2013) Anti-inflammatory properties rather than anti-oxidant capability is the major mechanism of neuroprotection by sodium salicylate in a chronic rotenone model of Parkinson’s disease. Neuroscience 231:420–431
Thangarajan S, Deivasigamani A, Natarajan SS, Krishnan P, Mohanan SK (2014) Neuroprotective activity of L-theanine on 3-nitropropionic acid-induced neurotoxicity in rat striatum. Int J Neurosci. 124:673
Velloso NA, Dalmolin GD, Fonini G, Gindri Sinhorin VD, Ferreira da Silveira A, Rubin MA, et al (2008) Spermine attenuates behavioral and biochemical alterations induced by quinolinic acid in the striatum of rats. Brain Res 1198:107–114
Wills E (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99:667–676
Acknowledgments
Authors are thankful to Science and Engineering Board (SERB), Department of Science and Technology, Govt. of India, New Delhi for providing financial assistance under Fast Track Scheme (DST-SERB-FTYS) to Dr. Puneet Kumar. The Junior Research Fellowship to Mr. Sumit Jamwal is also highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jamwal, S., Singh, S., Kaur, N. et al. Protective Effect of Spermidine Against Excitotoxic Neuronal Death Induced by Quinolinic Acid in Rats: Possible Neurotransmitters and Neuroinflammatory Mechanism. Neurotox Res 28, 171–184 (2015). https://doi.org/10.1007/s12640-015-9535-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-015-9535-y