Abstract
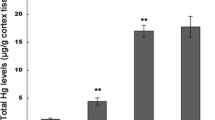
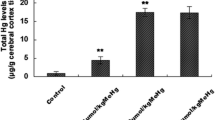
Methylmercury (MeHg) is a ubiquitous environmental toxin that causes neurologic and developmental diseases. Oxidative damage and excitotoxicity are putative mechanisms, which underlie MeHg-induced neurotoxicity. In this study, the cross-talk between the oxidative damage and excitotoxicity pathways and the protective effects of riluzole in the rat cortex were explored. Rats were injected with MeHg and/or riluzole, and cold vapor atomic fluorescence spectrometry, hematoxylin and eosin staining, flow cytometry, fluorescence assays, spectrophotometry, real-time PCR, and Western blotting were used to evaluate neurotoxicity. The present study showed that (1) MeHg accumulated in the cerebral cortex and caused pathology. (2) MeHg caused oxidative damage by inducing glutathione (GSH) depletion, reactive oxygen species (ROS) production, inhibition of antioxidant enzyme activity, and alteration of the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling. (3) MeHg disrupted the glutamate transporters (GluTs), glutamate–glutamine cycle, and N-methyl-d-aspartate receptor expression and induced excitotoxicity. (4) Excitotoxicity resulted in disruption of GSH synthesis, calcium overloading, oxidative damage, and excessive ROS production. (5) Pretreatment with riluzole antagonized MeHg neurotoxicity by down regulating cross-talk between the oxidative damage and excitotoxicity pathways. In conclusion, the cross-talk between the oxidative damage and excitotoxicity pathways caused by MeHg exposure was linked by GluTs and calcium and inhibited by riluzole treatment.









Similar content being viewed by others
Abbreviations
- MeHg:
-
Methylmercury
- CNS:
-
Central nervous system
- GSH:
-
Glutathione
- ROS:
-
Reactive oxygen species
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- CNC:
-
Cap-n-collar
- bZIP:
-
Basic region leucine zipper
- AREs:
-
Antioxidant response elements
- HO-1:
-
Heme oxygenase 1
- γ-GCS:
-
γ-Glutamylcysteine synthetase
- GPx-1:
-
Glutathione peroxidase 1
- Glu:
-
Glutamate
- NMDA:
-
N-methyl-d-aspartate
- Gln:
-
Glutamine
- PAG:
-
Phosphate activated glutaminase
- GluTs:
-
Glutamate transporters
- GLAST:
-
Glutamate/aspartate transporter
- GLT-1:
-
Glutamate transporter-1
- GS:
-
Glutamine synthetase
- H2O2 :
-
Hydrogen peroxide
- ONOO− :
-
Peroxynitrite
- ALS:
-
Amyotrophic lateral sclerosis
- SOD:
-
Superoxide dismutase
- GSH-Px:
-
Glutathione peroxidase
- DCFH-DA:
-
2, 7-Dichlorofluorescin-diacetate
- BSA:
-
Bovine serum albumin
- CVAFS:
-
Cold vapor atomic fluorescence spectrometry
- HE:
-
Hematoxylin and eosin
- DTNB:
-
1, 2-Dithio-bis nitro benzoic acid
- PVDF:
-
Polyvinylidene difluoride
- ANOVA:
-
One-way analysis of variance
- BBB:
-
Blood–brain barrier
- LAT:
-
L-type neutral amino acid carrier transport
References
Ahamed M, Akhtar MJ, Siddiqui MA et al (2011) Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 283(2–3):101–108
Allgaier C (2002) Ethanol sensitivity of NMDA receptors. Neuroche Int 41(6):377–382
Aschner M, Clarkson TW (1987) Mercury 203 distribution in pregnant and nonpregnant rats following systemic infusions with thiol-containing amino acids. Teratology 36(3):321–328
Bensimon G, Lacomblez L, Meininger V (1994) A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/riluzole study group. N Engl J Med 330(9):585–591
Castoldi AF, Coccini T, Ceccatelli S et al (2001) Neurotoxicity and molecular effects of methylmercury. Brain Res Bull 55(2):197–203
Chan E, Yung WH, Baumann KI (1996) Cytoplasmic Ca2+ concentrations in intact Merkel cells of an isolated, functioning rat sinus hair preparation. Exp Brain Res 108(3):357–366
Curi TC, De Melo MP, De Azevedo RB et al (1997) Glutamine utilization by rat neutrophils: presence of phosphate-dependent glutaminase. Am J Physiol 273(4 Pt 1):1124–1129
Deng Y, Xu Z, Xu B et al (2009) The protective effect of riluzole on manganese caused disruption of glutamate-glutamine cycle in rats. Brain Res 1289:106–117
Distelmaier F, Valsecchi F, Forkink M et al (2012) Trolox-sensitive reactive oxygen species regulate mitochondrial morphology, oxidative phosphorylation and cytosolic calcium handling in healthy cells. Antioxid Redox Signal 17(12):1657–1669
Farina M, Aschner M, Rocha JB (2011) Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol 256(3):405–417
Franco R, Cidlowski JA (2012) Glutathione efflux and cell death. Antioxid Redox Signal 17(12):1694–1713
Fumagalli E, Funicello M, Rauen T (2008) Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol 578(2–3):171–176
Gabryel B, Małecki A (2006) Ebselen attenuates oxidative stress in ischemic astrocytes depleted of glutathione. Comparison with glutathione precursors. Pharmacol Rep 58(3):381–392
Gyengesi E, Paxinos G, Andrews ZB (2012) Oxidative stress in the hypothalamus: the importance of calcium signaling and mitochondrial ROS in body weight regulation. Curr Neuropharmacol 10(4):344–353
Had-Aissouni L (2012) Toward a new role for plasma membrane sodium-dependent glutamate transporters of astrocytes: maintenance of antioxidant defenses beyond extracellular glutamate clearance. Amino Acids 42(1):181–197
He Y, Cui J, Lee JC et al (2011) Prolonged exposure of cortical neurons to oligomeric amyloid-β impairs NMDA receptor function via NADPH oxidase-mediated ROS production: protective effect of green tea (-)-epigallocatechin-3-gallate. ASN Neuro 3(1):e00050
Hong YS, Kim YM, Lee KE (2012) Methylmercury exposure and health effects. J Prev Med Public Health 45(6):353–363
Itoh K, Tong KI, Yamamoto M (2004) Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 36(10):1208–1213
Jaekel B, Mühlberg K, Garcia de Arriba S et al (2006) Neuroprotection associated with alternative splicing of NMDA receptors in rat cortical neurons. Br J Pharmacol 147(6):622–633
Johnson FO, Yuan Y, Hajela RK et al (2011) Exposure to an environmental neurotoxicant hastens the onset of amyotrophic lateral sclerosis-like phenotype in human Cu2+/Zn2+ superoxide dismutase 1 G93A mice: glutamate-mediated excitotoxicity. J Pharmacol Exp Ther 338(2):518–527
Jyrkkänen HK, Kuosmanen S, Heinäniemi M et al (2011) Novel insights into the regulation of antioxidant-response-element-mediated gene expression by electrophiles: induction of the transcriptional repressor BACH1 by Nrf2. Biochem J 440(2):67–174
Kolls JK (2006) Oxidative stress in sepsis: a redox redux. J Clin Invest 116(4):860–863
Liu Y, Wong TP, Aarts M et al (2007) NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci 27(11):2846–2857
Liu CH, Jiao H, Guo ZH et al (2013) Up-regulated GLT-1 resists glutamate toxicity and attenuates glutamate-induced calcium loading in cultured neurocytes. Basic Clin Pharmacol Toxicol 112(1):19–24
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Mehta A, Prabhakar M, Kumar P et al (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698(1–3):6–18
Meldrum BS (2000) Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 130(4S Suppl):1007S–1015S
Muller A, Maurin L, Bonne C (1998) Free radicals and glutamate uptake in the retina. Gen Pharmacol 30(3):315–318
Murali G, Panneerselvam KS, Panneerselvam C (2008) Age-associated alterations of lipofuscin, membrane-bound ATPases and intracellular calcium in cortex, striatum and hippocampus of rat brain: protective role of glutathione monoester. Int J Dev Neurosci 26(2):211–215
Ni M, Li X, Yin Z et al (2010) Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol Sci 116(2):590–603
Polunas M, Halladay A, Tjalkens RB et al (2011) Role of oxidative stress and the mitochondrial permeability transition in methylmercury cytotoxicity. Neurotoxicology 32(5):526–534
Rastogi RP, Singh SP, Häder DP et al (2010) Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Res Commun 397(3):603–607
Renis M, Cardile V, Russo A et al (1998) Glutamine synthetase activity and HSP70 levels in cultured rat astrocytes: effect of 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine. Brain Res 783(1):143–150
Rinwa P, Jaggi AS, Singh N (2012) Pharmacological investigation of memory restorative effect of riluzole in mice. Indian J Pharmacol 44(3):366–371
Roos D, Seeger R, Puntel R et al (2012) Role of calcium and mitochondria in MeHg-mediated cytotoxicity. J Biomed Biotechnol 2012:248764
Shen J (2013) Modeling the glutamate-glutamine neurotransmitter cycle. Front Neuroenergetics 5:1
Stockwell PB, Corns WT (1993) The role of atomic fluorescence spectrometry in the automatic environmental monitoring of trace element analysis. J Automatic Chem 15(3):79–84
Storch A, Burkhardt K, Ludolph AC et al (2000) Protective effects of riluzole on dopamine neurons: involvement of oxidative stress and cellular energy metabolism. J Neurochem 75(6):2259–2269
Thörn I, Olsson-Strömberg U, Ohlsen C et al (2005) The impact of RNA stabilization on minimal residual disease assessment in chronic myeloid leukemia. Haematologica 90(11):1471–1476
Toyama T, Sumi D, Shinkai Y et al (2007) Cytoprotective role of Nrf2/Keap1 system in methylmercury toxicity. Biochem Biophys Res Commun 363(3):645–650
Trotti D, Rossi D, Gjesdal O et al (1996) Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem 271(11):5976–5979
Vahter ME, Mottet NK, Friberg LT et al (1995) Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol Appl Pharmacol 134(2):273–284
Villalba M, Martínez-Serrano A, Börner C et al (1992) NMDA-induced increase in [Ca2+]i and 45Ca2+ uptake in acutely dissociated brain cells derived from adult rats. Brain Res 570(1–2):347–353
Zarate CA, Manji HK (2008) Riluzole in psychiatry: a systematic review of the literature. Expert Opin Drug Metab Toxicol 4(9):1223–1234
Acknowledgments
A grant from the National Natural Science Foundation of China (No. 81172631) supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, Y., Xu, Z., Xu, B. et al. Exploring Cross-Talk Between Oxidative Damage and Excitotoxicity and the Effects of Riluzole in the Rat Cortex After Exposure to Methylmercury. Neurotox Res 26, 40–51 (2014). https://doi.org/10.1007/s12640-013-9448-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-013-9448-6