Abstract
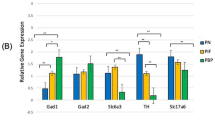
The development of the central nervous system can be permanently affected by insults received during the perinatal period, predisposing the organism to long-term behavioral and neurochemical abnormalities. Rats exposed to different types of stress during the last week of gestation produce offspring that show several alterations, many of which have been attributed to changes in dopamine (DA) neurotransmission that could serve as the neurochemical basis for the development of neuropsychiatric disorders. Employing an immunocytochemical approach, we studied the expression levels of two transcription factors Nurr1 and Pitx3 which are expressed at critical moments of DA neurons differentiation as well as the expression of the rate limiting enzyme in DA synthesis, tyrosine hydroxylase (TH) in mesencephalic areas of the brains of prenatally stressed (PS) offspring at different postnatal ages. Main results show that stress exerted to the gestant mother produces permanent effect in the ontogenetic expression of key factors related to the DA metabolism mainly in the ventral tegmental area (VTA) of the mesencephalon. The immunocytochemical expression of the transcription factor Nurr1 shows an increase at postnatal days (PNDs) 7, 28, and 60 whereas Pitx3 shows a decrease at PND 28 and an increase at 60 PND. The rate limiting step in DA synthesis, the enzyme TH shows a decrease at PND 7 to reach control levels at PNDs 28 and 60. The increase of TFs might be up-regulating TH in order to restore DA levels that were previously seen to be normal before puberty. The area selectivity of the increase of the TFs toward VTA and the mesolimbic pathway indicates that an insult received during the prenatal period will exert mainly motivational, emotional, and reward behavior impairments in the adult life.









Similar content being viewed by others
References
Adrover E, Berger MA, Perez AA, Tarazi FI, Antonelli MC (2007) Effects of prenatal stress on dopamine D2 receptor asymmetry in rat brain. Synapse 61:459–462
Alavian KN, Scholz C, Simon HH (2008) Transcriptional regulation of mesencephalic dopaminergic neurons: the full circle of life and death. Mov Disord 23:319–328
Alonso SJ, Navarro E, Santana C, Rodriguez M (1997) Motor lateralization, behavioral despair and dopaminergic brain asymmetry after prenatal stress. Pharmacol Biochem Behav 58:443–448
Aranda A, Pascual A (2001) Nuclear hormone receptors and gene expression. Physiol Rev 81:1269–1304
Balda MA, Anderson KL, Itzhak Y (2009) The neuronal nitric oxide synthase (nNOS) gene contributes to the regulation of tyrosine hydroxylase (TH) by cocaine. Neurosci Lett 457:120–124
Barros VG, Boado LA, Antonelli MC (2002) Postnatal ontogeny of rat brain D2 dopamine receptors in a prenatal stress model. Program 274.1, abstract viewer/itenerary planner. Society for Neuroscience, Washington, DC. Available on CD-Rom
Barros VG, Duhalde-Vega M, Caltana L, Brusco A, Antonelli MC (2006) Astrocyte-neuron vulnerability to prenatal stress in the adult rat brain. J Neurosci Res 83:787–800
Berger MA, Barros VG, Sarchi MI, Tarazi FI, Antonelli MC (2002) Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochem Res 27:1525–1533
Boksa P, El-Khodor BF (2003) Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: possible implications for schizophrenia and other disorders. Neurosci Biobehav Rev 27:91–101
Casolini P, Domenici MR, Cinque C, Alema GS, Chiodi V, Galluzzo M, Musumeci M, Mairesse J, Zuena AR, Matteucci P, Marano G, Maccari S, Nicoletti F, Catalani A (2007) Maternal exposure to low levels of corticosterone during lactation protects the adult offspring against ischemic brain damage. J Neurosci 27:7041–7046
Chapillon P, Patin V, Roy V, Vincent A, Caston J (2002) Effects of pre- and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: a review. Dev Psychobiol 41:373–387
Chung S, Hedlund E, Hwang M, Kim DW, Shin BS, Hwang DY, Jung Kang U, Isacson O, Kim KS (2005) The homeodomain transcription factor Pitx3 facilitates differentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Mol Cell Neurosci 28:241–252
Darnaudery M, Maccari S (2008) Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev 57:571–585
Del Arco A, Mora F (2009) Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J Neural Transm 116:941–952
Diaz R, Ogren SO, Blum M, Fuxe K (1995) Prenatal corticosterone increases spontaneous and d-amphetamine induced locomotor activity and brain dopamine metabolism in prepubertal male and female rats. Neuroscience 66:467–473
Diaz R, Fuxe K, Ogren SO (1997) Prenatal corticosterone treatment induces long-term changes in spontaneous and apomorphine-mediated motor activity in male and female rats. Neuroscience 81:129–140
Fride E, Weinstock M (1988) Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci 42:1059–1065
Fride E, Weinstock M (1989) Alterations in behavioral and striatal dopamine asymmetries induced by prenatal stress. Pharmacol Biochem Behav 32:425–430
Fujisawa H, Okuno S (2005) Regulatory mechanism of tyrosine hydroxylase activity. Biochem Biophys Res Commun 338:271–276
Guillot TS, Miller GW (2009) Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol 39:149–170
Hagerty T, Morgan WW, Elango N, Strong R (2001) Identification of a glucocorticoid-responsive element in the promoter region of the mouse tyrosine hydroxylase gene. J Neurochem 76:825–834
Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, Le Moal M, Demotes-Mainard J (1995) Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens. Brain Res 685:179–186
Hermanson E, Borgius L, Bergsland M, Joodmardi E, Perlmann T (2006) Neuropilin1 is a direct downstream target of Nurr1 in the developing brain stem. J Neurochem 97:1403–1411
Huizink AC, Mulder EJ, Buitelaar JK (2004) Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility? Psychol Bull 130:115–142
Jacobs FM, van Erp S, van der Linden AJ, von Oerthel L, Burbach JP, Smidt MP (2009) Pitx3 potentiates Nurr1 in dopamine neuron terminal differentiation through release of SMRT-mediated repression. Development 136:531–540
Katunar MR, Adrover E, Barros VG, Saez T, Silvagni A, Brusco A, Carboni E, Antonelli MC (2008) Dopaminergic neurotransmission in a rat model of prenatal stress. Program No. 477.13. 2008 neuroscience meeting planner. Society for Neuroscience, Washington, DC
Katunar MR, Saez T, Brusco A, Antonelli MC (2009) Immunocytochemical expression of dopamine-related transcription factors Pitx3 and Nurr1 in prenatally stressed adult rats. J Neurosci Res 87:1014–1022
Kendler KS, Karkowski LM, Prescott CA (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841
Lewis DA, Levitt P (2002) Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25:409–432
Lopez-Figueroa MO, Itoi K, Watson SJ (1998) Regulation of nitric oxide synthase messenger RNA expression in the rat hippocampus by glucocorticoids. Neuroscience 87:439–446
Luo Y, Henricksen LA, Giuliano RE, Prifti L, Callahan LM, Federoff HJ (2007) VIP is a transcriptional target of Nurr1 in dopaminergic cells. Exp Neurol 203:221–232
Maccari S, Morley-Fletcher S (2007) Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology 32(Suppl 1):S10–S15
Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M (1995) Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci 15:110–116
Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O (2003) Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev 27:119–127
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839
McArthur S, McHale E, Dalley JW, Buckingham JC, Gillies GE (2005) Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J Neuroendocrinol 17:475–482
McArthur S, McHale E, Gillies GE (2007) The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex- region- and time-specific manner. Neuropsychopharmacology 32:1462–1476
McClure WO, Ishtoyan A, Lyon M (2004) Very mild stress of pregnant rats reduces volume and cell number in nucleus accumbens of adult offspring: some parallels to schizophrenia. Brain Res Dev Brain Res 149:21–28
Nagatsu T, Ichinose H (1999) Molecular biology of catecholamine-related enzymes in relation to Parkinson’s disease. Cell Mol Neurobiol 19:57–66
Otten U, Thoenen H (1976) Selective induction of tyrosine hydroxylase and dopamine beta-hydroxylase in sympathetic ganglia in organ culture: role of glucocorticoids as modulators. Mol Pharmacol 12:353–361
Paxinos G, Watson C (1986) The rat brain in stereotaxis coordinates. Academic Press, San Diego
Perlmann T, Wallen-Mackenzie A (2004) Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res 318:45–52
Prakash N, Wurst W (2006) Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci 63:187–206
Reznikov AG, Nosenko ND, Tarasenko LV, Sinitsyn PV, Polyakova LI (2001) Early and long-term neuroendocrine effects of prenatal stress in male and female rats. Neurosci Behav Physiol 31:1–5
Sanchez HL, Silva LB, Portiansky EL, Herenu CB, Goya RG, Zuccolilli GO (2008) Dopaminergic mesencephalic systems and behavioral performance in very old rats. Neuroscience 154:1598–1606
Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP, Conneely OM (1998) Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci USA 95:4013–4018
Schultz W (1997) Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol 7:191–197
Schwarz PM, Gierten B, Boissel JP, Forstermann U (1998) Expressional down-regulation of neuronal-type nitric oxide synthase I by glucocorticoids in N1E-115 neuroblastoma cells. Mol Pharmacol 54:258–263
Semina EV, Reiter RS, Murray JC (1997) Isolation of a new homeobox gene belonging to the Pitx/Rieg family: expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet 6:2109–2116
Silvagni A, Barros VG, Mura C, Antonelli MC, Carboni E (2008) Prenatal restraint stress differentially modifies basal and stimulated dopamine and noradrenaline release in the nucleus accumbens shell: an ‘in vivo’ microdialysis study in adolescent and young adult rats. Eur J Neurosci 28:744–758
Smidt MP, van Schaick HS, Lanctot C, Tremblay JJ, Cox JJ, van der Kleij AA, Wolterink G, Drouin J, Burbach JP (1997) A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc Natl Acad Sci USA 94:13305–13310
Smidt MP, Asbreuk CH, Cox JJ, Chen H, Johnson RL, Burbach JP (2000) A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci 3:337–341
Smidt MP, Smits SM, Burbach JP (2003) Molecular mechanisms underlying midbrain dopamine neuron development and function. Eur J Pharmacol 480:75–88
Sousa KM, Mira H, Hall AC, Jansson-Sjostrand L, Kusakabe M, Arenas E (2007) Microarray analyses support a role for Nurr1 in resistance to oxidative stress and neuronal differentiation in neural stem cells. Stem Cells 25:511–519
Swanson J, Castellanos FX, Murias M, LaHoste G, Kennedy J (1998) Cognitive neuroscience of attention deficit hyperactivity disorder and hyperkinetic disorder. Curr Opin Neurobiol 8:263–271
Verheij MM, Cools AR (2008) Twenty years of dopamine research: individual differences in the response of accumbal dopamine to environmental and pharmacological challenges. Eur J Pharmacol 585:228–244
Volpicelli F, Caiazzo M, Greco D, Consales C, Leone L, Perrone-Capano C, Colucci D’Amato L, di Porzio U (2007) Bdnf gene is a downstream target of Nurr1 transcription factor in rat midbrain neurons in vitro. J Neurochem 102:441–453
Wallen A, Zetterstrom RH, Solomin L, Arvidsson M, Olson L, Perlmann T (1999) Fate of mesencephalic AHD2-expressing dopamine progenitor cells in NURR1 mutant mice. Exp Cell Res 253:737–746
Ward IL, Weisz J (1984) Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology 114:1635–1644
Weinstock M (2001) Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol 65:427–451
Weinstock M (2002) Can the behaviour abnormalities induced by gestational stress in rats be prevented or reversed? Stress 5:167–176
Weinstock M (2005) The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun 19:296–308
Weinstock M (2008) The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 32:1073–1086
Acknowledgments
We are greatly indebted to Dr. Marten Smidt (Utrecht University, The Netherlands) for the gift of Pitx3 antibody. We thank Dr. Carlos J. Baier for his help in preparing the figures and for critical reading of the manuscript and helpful discussions. We appreciate the help of Dr. Alberto J. Ramos in the image analysis of the data. The skillful technical assistance and bibliographical management of Mrs. Susana Buglione is greatly appreciated. This research was supported by grants from CONICET (PIP 6015) and ANPCYT (PICT 31981) (to MCA) and UBACYT M004 (to AB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katunar, M.R., Saez, T., Brusco, A. et al. Ontogenetic Expression of Dopamine-Related Transcription Factors and Tyrosine Hydroxylase in Prenatally Stressed Rats. Neurotox Res 18, 69–81 (2010). https://doi.org/10.1007/s12640-009-9132-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9132-z