Abstract
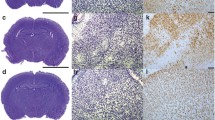
Ischemic brain injury is a dynamic process that involves oxidative stress, inflammation, and cell death, as well as activation of endogenous adaptive and regenerative mechanisms depending on activation of transcription factors such as hypoxia inducible factor 1-alpha (HIF-1α). Because CoCl2 activates HIF-1α, we described a new focal-hypoxia model by direct intracerebral CoCl2 injection. Adult male Wistar rats were intracerebrally injected with CoCl2 (2 μl–50 mM), in frontoparietal cortex of right hemisphere, and saline (2 μl) in the contralateral hemisphere. In slides of fixed brains at 1, 6, 9, 24 h or 5 day after treatment, TTC, histochemistry (toluidine blue, Hoescht-33342, TUNEL), immunostaining (HIF-1α, GFAP), Lycopersicon esculentum lectin staining, and electron microscopy (EM) were performed. Immediately after 1 h post CoCl2 injection, HIF-1α stabilization and neuronal nuclear shrinkage and cromathin condensation were observed by immunostaining and EM, respectively. Neuronal apoptotic nuclear morphology and GFAP immunoreactivity and lectin maximal reactivity were detected during 6–9 h. Ultrastructural alterations of morphology included edematous perinuclear cytoplasm, organelles and endoplasmic reticulum (RE) enlargement, mitochondrial swelling with increased matrix density, and deposits of electron-dense material. Neurons showed particular nuclear indentations. Astrocytes and oligodendrocytes presented alterations in both nuclei and RE with dilated lumen and altered mitochondrias, and all these ultrastructural changes became detectable at day 5. CoCl2 cortical injection mimics focal brain ischemia, inducing neuronal death and glial activation. This model brings the opportunity to develop focal ischemia in selected brain areas to study their functional consequences and potential pharmacological therapies for in vivo models of stroke.






Similar content being viewed by others
References
Al-Abdulla NA, Portera-Cailliau C, Martin LJ (1998) Occipital cortex ablation in adult rat causes retrograde neuronal death in the lateral geniculate nucleus that resembles apoptosis. Neuroscience 86:191–209
Berra E, Ginouvès A, Pouysségur J (2006) The hypoxia-inducible-factor hydroxylases bring fresh air into hypoxia signalling. EMBO Rep 7:41–45
Caltana LR, Merelli A, Ramos A, Lopez L, Lazarowski A, Brusco H (2008) Intranasal administration of Human Recombinant Erythropoietin (rHuEpo) improves the spontaneous motor activity in hypoxic rats in spite of brain MDR-1 overexpression. Program No.552.16 2008. Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington, DC, Online
Carmichael ST (2005) Rodent models of focal stroke: size, mechanism and purpose. NeuroRx 2:396–409
Chen XQ, Wang SJ, Du JZ, Chen XC (2007) Diversities in hepatic HIF-1, IGF-I/IGFBP-1, LDH/ICD, and their mRNA expressions induced by CoCl2 in Qinghai-Tibetan plateau mammals and sea level mice. Am J Physiol Regul Integr Comp Physiol 292:R516–R526
Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP (2002) Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62:3387–3394
del Zoppo GJ (2006) Stroke and neurovascular protection. N Engl J Med 354:553–555
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54
Gibson CL, Coughlan TC, Murphy SP (2005) Glial nitric oxide and ischemia. Glia 50:417–426
Hara A, Niwa M, Aoki H, Kumada M, Kunisada T, Oyama T (2006) A new model of retinal photoreceptor cell degeneration induced by a chemical hypoxia-mimicking agent, cobalt chloride. Brain Res 1109:192–200
Ignácio AR, Muller YMR, Carvalho MSL, Nazari EM (2005) Distribution of microglial cells in the cerebral hemispheres of embryonic and neonatal chicks. Braz J Med Biol Res 38:1615–1622
Jones NM, Liubov D, Callaway JK, Lee E, Beart PM (2008) Long-Term functional and protective actions of preconditioning with hypoxia, cobalt chloride, and desferrioxamine against hypoxic-ischemic injury in neonatal rats. Pediatr Res 63:620–624
Karovic O, Tonazzini I, Rebola N, Edström E, Lövdahl C, Fredholm BB, Daré E (2007) Toxic effects of cobalt in primary cultures of mouse astrocytes. Similarities with hypoxia and role of HIF-1alpha. Biochem Pharmacol 73:694–708
Kaur C, Sivakumar V, Zhang Y, Ling EA (2006) Hypoxia-induced astrocytic reaction and increased vascular permeability in the rat cerebellum. Glia 54:826–839
Lazarowski A, Caltana L, Merelli A, Rubio MD, Ramos AJ, Brusco A (2007) Neuronal mdr-1 gene expression after experimental focal hypoxia: a new obstacle for neuroprotection? J Neurol Sci 258:84–92
Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, Shuaib A, Hårdemark HG, Wasiewski WW (2006) Stroke-Acute Ischemic NXY Treatment (SAINT I) Trial Investigators. NXY-059 for acute ischemic stroke. N Engl J Med 354:588–600
Olson EE, McKeon RJ (2004) Characterization of cellular and neurological damage following unilateral hypoxia/ischemia. J Neurol Sci 227:7–19
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, San Diego
Ramos AJ, Rubio MD, Defagot C, Hischberg L, Villar MJ, Brusco A (2004) The 5HT1A receptor agonist, 8-OH-DPAT, protects neurons and reduces astroglial reaction after ischemic damage caused by cortical devascularization. Brain Res 1030:201–220
Ratan RR, Siddiq A, Smirnova N, Karpisheva K, Haskew-Layton R, McConoughey S, Langley B, Estevez A, Huerta PT, Volpe B, Roy S, Sen CK, Gazaryan I, Cho S, Fink M, LaManna J (2007) Harnessing hypoxic adaptation to prevent, treat, and repair stroke. J Mol Med 85:1331–1338
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Semenza GL (2002a) Physiology meets biophysics: visualizing the interaction of hypoxia-inducible factor 1 alpha with p300 and CBP. Proc Natl Acad Sci USA 99:11570–11572
Semenza GL (2002b) HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 8:62–67
Tagliaferro P, Ramos AJ, López EM, Pecci Saavedra J, Brusco A (1997) Neural and astroglial effects of a chronic parachlorophenylalanine-induced serotonin synthesis inhibition. Mol Chem Neuropathol 32:195–211
Wang G, Hazra TK, Mitra S, Lee HM, Englander EW (2000) Mitochondrial DNA damage and hypoxic response are induced by CoCl2 in rat neuronal PC12 cells. Nucleic Acids Res 28:2135–2140
Yuan Y, Hilliard G, Ferguson T, Millhorn DE (2003) Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem 278:15911–15916
Acknowledgments
We thank Mrs. Emerita Jorge Vilela de Bianchieri for her expert technical assistance with the EM studies and Dr. A. Ramos for his help in the interpretation of the results. Sources of Funding: Supported by grants UBACYT M-072, UBACYT M-004, CONICET PIP5034 (to A.B.).
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Lazarowski and A. Brusco contributed equally to this work.
Rights and permissions
About this article
Cite this article
Caltana, L., Merelli, A., Lazarowski, A. et al. Neuronal and Glial Alterations Due to Focal Cortical Hypoxia Induced by Direct Cobalt Chloride (CoCl2) Brain Injection. Neurotox Res 15, 348–358 (2009). https://doi.org/10.1007/s12640-009-9038-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9038-9