Abstract
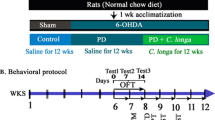
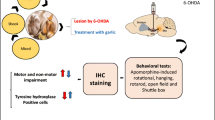
Mucuna pruriens (MP) has long been used in Indian traditional medicine as support in the treatment of Parkinson’s disease. However, no systematic preclinical studies that aimed at evaluating the efficacy of this substance are available to date. This study undertook an extensive evaluation of the antiparkinsonian effects of an extract of MP seeds known to contain, among other components, 12.5% l-dihydroxyphenylalanine (l-DOPA), as compared to equivalent doses of l-DOPA. Moreover, the neuroprotective efficacy of MP and its potential rewarding effects were evaluated. The results obtained reveal how an acute administration of MP extract at a dose of 16 mg/kg (containing 2 mg/kg of l-DOPA) consistently antagonized the deficit in latency of step initiation and adjusting step induced by a unilateral 6-hydroxydopamine lesion, whereas l-DOPA was equally effective only at the doses of 6 mg/kg. At the same dosage, MP significantly improved the placement of the forelimb in vibrissae-evoked forelimb placing, suggesting a significant antagonistic activity on both motor and sensory-motor deficits. The effects of MP extract were moreover investigated by means of the turning behavior test and in the induction of abnormal involuntary movements (AIMs) after either acute or subchronic administration. MP extract acutely induced a significantly higher contralateral turning behavior than l-DOPA (6 mg/kg) when administered at a dose of 48 mg/kg containing 6 mg/kg of l-DOPA. On subchronic administration, both MP extract (48 mg/kg) and l-DOPA (6 mg/kg) induced sensitization of contralateral turning behavior; however, l-DOPA alone induced a concomitant sensitization in AIMs suggesting that the dyskinetic potential of MP is lower than that of l-DOPA. MP (48 mg/kg) was also effective in antagonizing tremulous jaw movements induced by tacrine, a validated test reproducing parkinsonian tremor. Furthermore, MP induced no compartment preference in the place preference test, indicating the lack of components characterized by rewarding effects in the extract. Finally, in a subchronic mice model of 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine hydrochloride (MPTP)-induced dopamine neuron degeneration, MP extract did not prove capable of preventing either tyrosine hydroxylase decrease induced by MPTP or astroglial or microglial activation as assessed by means of GFAP and CD11b immunohistochemistry, supporting the absence of neuroprotective effects by MP. Characterization MP extract strongly supports its antiparkinsonian activity.







Similar content being viewed by others
References
Bishop C, Taylor JL, Kuhn DM, Eskow KL, Park JY, Walker PD (2006) MDMA and fenfluramine reduce L-DOPA-induced dyskinesia via indirect 5-HT1A receptor stimulation. Eur J Neurosci 23:2669–2676
Carta AR, Pinna A, Morelli M (2006) How reliable is the behavioural evaluation of dyskinesia in animal models of Parkinson’s disease? Behav Pharmacol 17:393–402
Carta M, Carlsson T, Kirik D, Björklund A (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833
Carta AR, Frau L, Pinna A, Pontis S, Simola N, Schintu N, Morelli M (2008) Behavioral and biochemical, correlates of the dyskinetic potential of dopaminergic agonist in the 6-OHDA lesioned rat. Synapse 62:524–533
Chang JW, Wachtel SR, Young D, Kang UJ (1999) Biochemical and anatomical characterisation of forelimb adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. Neuroscience 88:617–628
Cousins MS, Carriero DL, Salamone JD (1997) Tremulous jaw movements induced by the acetylcholinesterase inhibitor tacrine: effects of antiparkinsonian drugs. Eur J Pharmacol 322:137–145
Henry B, Crossman AR, Brotchie JM (1998) Characterization of enhanced behavioral responses to L-DOPA following repeated administration in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Exp Neurol 151:334–342
Hussain G, Manyam BV (1997) Mucuna pruriens proves more effective than l-dopa in Parkinson’s disease animal model. Phytother Res 11:419–423
Ikeguchi K, Kuroda A (1995) Mianserin treatment of patients with psychosis induced by antiparkinsonian drugs. Eur Arch Psychiatry Clin Neurosci 244:320–324
Katzenschlager R, Evans A, Manson A, Patsalos PN, Ratnaraj N, Watt H, Timmerman L, Van der Giessen R, Lees AJ (2004) Mucuna pruriens in Parkinson’s disease: a double blind clinical and pharmacological study. J Neurosurg Psychiatry 75:1672–1677
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioral measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132
Manyam BV (1990) Paralysis agitans and levodopa in “Ayurveda”: ancient Indian medical treatise. Mov Disord 5:47–48
Manyam BV, Dhanasekaran M, Hare TA (2004) Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phytother Res 9:706–712
Marsden CD (1994) Parkinson’s disease. J Neurol Neurosurg Psychiatry 57:672–681
Meredith GE, Kang UJ (2006) Behavioral models of Parkinson’s disease in rodents: a new look at an old problem. Mov Disord 21:1595–1606
Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW (2000) Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci 23:S8–S19
Olanow CW, Damier P, Goetz CG, Mueller T, Nutt J, Rascol O, Serbanescu A, Deckers F, Russ H (2004) Multicenter, open-label, trial of sarizotan in Parkinson disease patients with levodopa-induced dyskinesias (the SPLENDID Study). Clin Neuropharmacol 27:58–62
Olsson M, Nikkhah G, Bentlage C, Bjorklund A (1995) Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci 15:3863–3875
Pellegrino LJ, Pellegrino AS, Cushman AJ (1979) A stereotaxic atlas of the rat brain. Plenum Press, New York
Pinna A, Pontis S, Morelli M (2006) Expression of dyskinetic movements and turning behaviour in subchronic L-DOPA 6-hydroxydopamine-treated rats is influenced by the testing environment. Behav Brain Res 171:175–178
Pinna A, Pontis S, Borsini F, Morelli M (2007) Adenosine A(2A) receptor antagonists improve deficits in initiation of movement and sensory motor integration in the unilateral 6-hydroxydopamine rat model of Parkinson’s disease. Synapse 61:606–614
Ravenscroft P, Chalon S, Brotchie JM, Crossman AR (2004) Ropinirole versus L-DOPA effects on striatal opioid peptide precursors in a rodent model of Parkinson’s disease: implications for dyskinesia. Exp Neurol 185:36–46
Salamone JD, Mayorga AJ, Trevitt JT, Cousins MS, Conlan A, Nawab A (1998) Tremulous jaw movements in rats: a model of parkinsonian tremor. Prog Neurobiol 56:591–611
Salamone JD, Carlson BB, Rios C, Lentini E, Correa M, Wisniecki A, Betz A (2005) Dopamine agonists suppress cholinomimetic-induced tremulous jaw movements in an animal model of Parkinsonism: tremorolytic effects of pergolide, ropinirole and CY 208-243. Behav Brain Res 156:173–179
Sathiyanarayanan L, Arulmozhi S (2007) Mucuna pruriens Linn—a comprehensive review. Pharmacogn Rev 1(1):157–162
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensiromotor outcome in unilateral rat model of stroke, cortical ablation, parkinsonism, and spinal cord injury. Neuropharmacology 39:777–787
Simola N, Fenu S, Baraldi PG, Tabrizi MA, Morelli M (2004) Blockade of adenosine A2A receptors antagonizes parkinsonian tremor in the rat tacrine model by an action on specific striatal regions. Exp Neurol 189:182–188
Sridhar KR, Bhat R (2007) Agrobotanical, nutritional and bioactive potential of unconventional legume—Mucuna. Livest Res Rural Dev 19:1–36. Article #126. Retrieved from http://www.cipav.org.co/lrrd/lrrd19/9/srid19126.htm
Tharakan B, Dhanasekaran M, Mize-Berge J, Manyam BV (2007) Anti-parkinson botanical Mucuna pruriens prevents levodopa induced plasmid and genomic DNA damage. Phytother Res 21:1124–1126
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Kasture and S. Pontis contributed equally to this study.
Rights and permissions
About this article
Cite this article
Kasture, S., Pontis, S., Pinna, A. et al. Assessment of Symptomatic and Neuroprotective Efficacy of Mucuna Pruriens Seed Extract in Rodent Model of Parkinson’s Disease. Neurotox Res 15, 111–122 (2009). https://doi.org/10.1007/s12640-009-9011-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9011-7