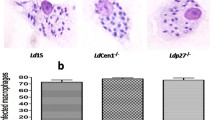
Abstract
Leishmaniasis is a serious global challenge with neither efficacious prophylactic vaccine nor effective and safe therapeutic measures. Cathelicidins, members of antimicrobial peptides family, are small proteins of innate immunity system, which represent a protective barrier against a number of potential pathogens in living organisms. The murine cathelicidin or cathelin-related antimicrobial peptide (CRAMP) is expressed by a variety of cells or tissues, and highly resembles to human cathelicidin (LL-37). It is naturally expressed at a low concentration in adolescent age, but extensively increases during cutaneous infections. Despite its important role, it has less been investigated in parasitic infections. Among all cells, macrophages and skin cells are the two important cells that directly have a relationship with Leishmania major parasites. The present study aimed to show whether cathelicidins protect their hosts following cutaneous leishmaniasis due to L. major parasites. Both in vitro and in vivo models of L. major infection were established by exposing of J744 cell line (murine macrophages) and BALB/c mice with the stationary phase of L. major promastigotes for 24 h and 7 days. The findings revealed that both macrophages and skin cells significantly (p < 0.05) expressed a high level of CRAMP gene and peptide after challenging with L. major parasites. Thus, our data suggest a protective role for cathelicidins against infections caused by L. major parasites. This experimental model could be considered as a novel potential vaccine candidate for planning future control strategy against human leishmaniasis.



Similar content being viewed by others
References
Agerberth B, Lee JY, Bergman T, Carlquist M, Boman HG, Mutt V et al (1991) Amino acid sequence of PR-39. Eur J Biochem 202(3):849–854
Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH (1995) FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci 92(1):195–199
Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J et al (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7(5):31
Assreuy J, Cunha FQ, Epperlein M, Noronha-Dutra A, O’Donnell CA, Liew FY et al (1994) Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major. Eur J Immunol 24(3):672–676
Bals R, Wilson J (2003) Cathelicidins-a family of multifunctional antimicrobial peptides. Cell Mol Life Sci CMLS 60(4):711–720
Bank E (2012) Leishmania major parasites and their interaction with human macrophages. Universitätsbibliothek Mainz
Bardan A, Nizet V, Gallo RL (2004) Antimicrobial peptides and the skin. Expert Opin Biol Ther 4(4):543–549
Cavalcante CS, Falcao CB, Fontenelle RO, Andreu D, Radis-Baptista G (2017) Anti-fungal activity of Ctn[15-34], the C-terminal peptide fragment of crotalicidin, a rattlesnake venom gland cathelicidin. J Antibiot 70(3):231–237
Clausen ML, Agner T (2016) Antimicrobial peptides, infections and the skin barrier. Curr Probl Dermatol 49:38–46
Daneshvar H, Sharifi I, Keyhani A, Tavakoli Kareshk A, Asadi A (2017) Comparative analysis of antimicrobial peptides gene expression in susceptible/resistant mice macrophages to Leishmania major infection. Middle East J Fam Med 7(10):18
Daneshvar H, Tavakoli Kareshk A, SharifiI KA, Tavakoli Oliaee R, Asadi A et al (2018) Host-parasite responses outcome regulate the expression of antimicrobial peptide genes in the skin of BALB/c and C57BL/6 murine strains following Leishmania major MRHO/IR/75/ER infection. Iran J Parasitol 13(4):515–523
DeGray G, Rajasekaran K, Smith F, Sanford J, Daniell H (2001) Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol 127(3):852–862
Desjeux P (1996) Leishmaniasis: public health aspects and control. Clin Dermatol 14(5):417–423
Diamond G, Beckloff N, Weinberg A, Kisich KO (2009) The roles of antimicrobial peptides in innate host defense. Curr Pharma Des 15(21):2377–2392
Do Nascimento VV, Mello ÉO, Carvalho LP, de Melo EJ, Carvalho AO, Fernandes KV et al (2015) PvD1 defensin, a plant antimicrobial peptide with inhibitory activity against Leishmania amazonensis. Biosci Rep 35(5):e00248
Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V et al (2001) Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Investig Dermatol 117(1):91–97
Dorschner RA, Lin KH, Murakami M, Gallo RL (2003) Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res 53(4):566–572
Dubos RJ (1939) Studies on a bactericidal agent extracted from a soil bacillus: I. Preparation of the agent. Its activity in vitro. J Exp Med 70(1):1
Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L et al (1997) Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem 272(20):13088–13093
Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3(9):710–720
Gordon YJ, Romanowski EG, McDermott AM (2005) A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res 30(7):505–515
Hailu A, Dagne DA, Boelaert M (2016a) Leishmaniasis. Negl Trop Dis-Sub-Saharan Africa. Springer, pp 87–112
Hailu T, Yimer M, Mulu W, Abera B (2016b) Challenges in visceral leishmaniasis control and elimination in the developing countries: a review. J Vector Borne Dis 53(3):193–198
Handman E, Bullen DV (2002) Interaction of Leishmania with the host macrophage. Trends Parasitol 18(8):332–334
Kao C, Lin X, Yi G, Zhang Y, Rowe-Magnus DA, Bush K (2016) Cathelicidin antimicrobial peptides with reduced activation of toll-like receptor signaling have potent bactericidal activity against colistin-resistant bacteria. MBio 7(5):01418-16
Karimkhani C, Wanga V, Naghavi P, Dellavalle RP, Naghavi M (2017) Global burden of cutaneous leishmaniasis. Lancet Infect Dis 17(3):264. https://doi.org/10.1016/s1473-3099(16)30217-1
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412
Kosciuczuk EM, Lisowski P, Jarczak J, Strzalkowska N, Jozwik A, Horbanczuk J et al (2012) Cathelicidins: family of antimicrobial peptides: a review. Mol Biol Rep 39(12):10957–10970
Machado LR, Ottolini B (2015) An evolutionary history of defensins: a role for copy number variation in maximizing host innate and adaptive immune responses. Front Immunol 6:115
Mello CP, Lima DB, Menezes RR, Bandeira IC, Tessarolo LD, Sampaio TL et al (2017) Evaluation of the antichagasic activity of batroxicidin, a cathelicidin-related antimicrobial peptide found in Bothrops atrox venom gland. Toxicon 130:56–62
Nizet V, Gallo RL (2003) Cathelicidins and innate defense against invasive bacterial infection. Scand J Infect Dis 35(9):670–676
Novoa Juiz V, Redondo Lucianez R (2016) Mucocutaneous leishmaniasis. Acta Otorrinolaringol Esp 67(6):16
Pivarcsi A, Nagy I, Kemeny L (2005) Innate immunity in the skin: how keratinocytes fight against pathogens. Curr Immunol Rev 1(1):29–42
Quinnell RJ, Courtenay O (2009) Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 136(14):1915–1934
Roby KD, Nardo AD (2013) Innate immunity and the role of the antimicrobial peptide cathelicidin in inflammatory skin disease. Drug Discov Today Dis Mech 10(3–4):e79–e82
Schlein Y (1993) Leishmania and sandflies: interactions in the life cycle and transmission. Parasitol Today 9(7):255–258
Shaw JJ (1994) Taxonomy of the genus Leishmania: present and future trends and their implications. Memórias do Instituto Oswaldo Cruz. 89(3):471–478
Tavakoli OR, Sharifi I, Afkar A, Tavakoli Kareshk A, Asadi A et al (2018) Unresponsiveness to meglumine antimoniate in anthroponotic cutaneous leishmaniasis field isolates: analysis of resistance biomarkers by gene expression profiling. Trop Med Int Health 23(6):622–633
Tavakoli OR, Sharifi I, Afkar A, Jafarzadeh A, Tavakoli Kareshk A et al (2019) Differential expression of TLRs 2, 4, 9, iNOS and TNF-α and arginase activity in peripheral blood monocytes from glucantime unresponsive and responsive patients with anthroponotic cutaneous leishmaniasis caused by Leishmania tropica. Microb Pathog 126:368–378
Vieira-Girao PR, Falcao CB, Rocha IR, Lucena HM, Costa FH, Radis-Baptista G (2017) Antiviral activity of Ctn[15-34], a cathelicidin-derived eicosapeptide, against infectious myonecrosis virus in litopenaeus vannamei primary hemocyte cultures. Food Environ Virol 16(10):017–9285
Yang YM, Guo YF, Zhang HS, Sun TY (2015) Antimicrobial peptide LL-37 circulating levels in chronic obstructive pulmonary disease patients with high risk of frequent exacerbations. J Thorac Dis 7(4):740–745
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci 84(15):5449–5453
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389–395
Zhao X, Wu H, Lu H, Li G, Huang Q (2013) LAMP: a database linking antimicrobial peptides. PLoS ONE 8(6):e66557
Acknowledgements
We are grateful from Kerman University of Medical Sciences, owing to finance supportive burden of this project and appreciatively the Leishmania Research Center due to use of experience and their requirements.
Funding
We did not receive any grants for the publication of this study.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
To work on animals, we received permission number IR.KMU.REC.1394.208 from the Ethical Review Board of Kerman University of Medical Sciences (Kerman, Iran).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asadi, A., Tavakoli Kareshk, A., Sharifi, I. et al. Murine cathelicidin: as a host defensive response against Leishmania major infection. J Parasit Dis 44, 633–638 (2020). https://doi.org/10.1007/s12639-020-01238-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-020-01238-0