Abstract
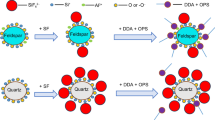
A novel green cationic surfactant Poly (propylene glycol) bis (2-aminopropyl ether) (PEA) with multiple amine groups was utilized as a collector for flotation separation of quartz from feldspar-quartz associated ore (FQA) without acid (particularly, HF) and alkali. The collecting performances and the adsorption mechanisms of PEA on the quartz/FQA were investigated through flotation experiments, ICP-OES, XRD, Zeta potential, FTIR and XPS measurements. A remarkable separation of the quartz from FQA has been realized in an environmental friendly way. The froth flotation results show that the flotation recovery of quartz is 97.79% and that of FQA is 19.30% at pH 9.00–9.50 with 10−4 M PEA when the particle size ranges from 150 to 270 μm. The results of batch flotation, ICP-OES and XRD analyses show that the first flotation product is almost quartz, while more feldspar and little quartz exist in the sink product when compared with the FQA feed. The mechanism of PEA acting as the collector for the separation of quartz/feldspar has been investigated. The PEA is adsorbed on the quartz surface by electrostatic and hydrogen bonding interaction with ─NH3+ /─NH2 head groups, while is less adsorbed on the feldspar surface due to electrostatic repulsion between the K+ / Na+ ions and the positively charged ─NH3+/─NH2 head groups of PEA. A conceptual adsorption model of the PEA cationic collector on the quartz and the FQA surfaces has been proposed.
Similar content being viewed by others
References
Tian J, Xu L, Wu H, Fang S, Deng W, Peng T, Sun W, Hu Y (2018) A novel approach for flotation recovery of spodumene, mica and feldspar from a lithium pegmatite ore. J Clean Prod 174:625–633
Vegliò F, Abbruzzese CBP (1999) Iron removal process for high-purity silica sands production by oxalic acid leaching. Ind Eng Chem Res:4443–4448
Vidyadhar A, Hanumantha Rao K (2007) Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system. J Colloid Interface Sci 306(2):195–204
Vatalis KI, Charalambides G, Benetis NP (2015) Market of high purity quartz innovative applications. Procedia Economics and Finance 24:734–742
Götze J (2009) Chemistry, textures and physical properties of quartz – geological interpretation and technical application. Mineral Mag 73(4):645–671
Bozkurt V, Ucbas Y (2000) Concentration of feldspar from trachyte. Mineral processing on the verge of the 21st century Balkema, Rotterdam, pp 327–329
Xu L, Tian J, Wu H, Deng W, Yang Y, Sun W, Gao Z, Hu Y (2017) New insights into the oleate flotation response of feldspar particles of different sizes: anisotropic adsorption model. J Colloid Interface Sci 505:500–508
Gaied ME, Gallala W (2015) Beneficiation of feldspar ore for application in the ceramic industry: influence of composition on the physical characteristics. Arab J Chem 8(2):186–190
Wills BA, Finch J (2015) Wills' mineral processing technology: an introduction to the practical aspects of ore treatment and mineral recovery. Butterworth-Heinemann
Tarleton ES (2014) Progress in filtration and separation. Academic Press
Larsen E, Kleiv RA (2016) Flotation of quartz from quartz-feldspar mixtures by the HF method. Miner Eng 98:49–51
Crundwell FK (2016) On the mechanism of the flotation of oxides and silicates. Miner Eng 95:185–196
Von Rybinski W, Schwuger M (1986) Adsorption of surfactant mixtures in froth flotation. Langmuir 2(5):639–643
Ata S, Jameson GJ (2005) The formation of bubble clusters in flotation cells. Int J Miner Process 76(1–2):123–139
Wang L, Liu R, Hu Y, Liu J, Sun W (2016) Adsorption behavior of mixed cationic/anionic surfactants and their depression mechanism on the flotation of quartz. Powder Technol 302:15–20
Sahoo H, Sinha N, Rath SS, Das B (2015) Ionic liquids as novel quartz collectors: insights from experiments and theory. Chem Eng J 273:46–54
O'meara R, Norman J, Hammond WE (1939) Froth flotation and agglomerate tabling of feldspars. Bull Am Ceram Soc 18(8):286–292
Sandvik K (1979) Flotation properties of feldspar and quartz. Minerals Research Laboratory
Shimoiizaka J, Nakatsuka K, Katayanagi T (1976) Separation of feldspar from quartz by a new flotation process. World Mining and Metals Technology Port City Press, Baltimore, pp 428–438
Crozier RD (1990) Non-metallic mineral flotation. Industrial Minerals 269:55–65
Demir C, GüLGONUL I, Bentli I, Çelik M (2003) Differential separation of albite from microcline by monovalent salts in HF medium. Miner Metall Process 20(3):120–124
Tang J, Sun B, Cheng Z, Chen X, Zhao H, Zhang S (1993) Theoretical studies and production practice of acidless and fluoless flotation of silica sand. Proceedings of XVIII international mineral Processinng congress, Sydney, Australia, pp 851–856
El-Salmawy M, Nakahiro Y, Wakamatsu T (1993) The role of alkaline earth cations in flotation separation of quartz from feldspar. Miner Eng 6(12):1231–1243
Vidyadhar A, Rao KH, Forssberg KSE (2002) Adsorption of N-tallow 1,3-Propanediamine–Dioleate collector on albite and quartz minerals, and selective flotation of albite from Greek Stefania feldspar ore. J Colloid Interface Sci 248(1):19–29
Chemical Safety Technology Description (2011) China Safety Data Sheet, Aladdin. http://new.aladdin-e.com:88/down/China/Aladdin-P108072%289046-10-0%29-v1.00_Poly%28propyleneglycol%29bis%282-aminopropylether%29.pdf. Accessed 11 Oct 2011
Zouboulis A, Avranas A (2000) Treatment of oil-in-water emulsions by coagulation and dissolved-air flotation. Colloids Surf A Physicochem Eng Asp 172(1–3):153–161
Li H, De Bruyn P (1966) Electrokinetic and adsorption studies on quartz. Surf Sci 5(2):203–220
Celik MS, Can I, Eren RH (1998) Removal of titanium impurities from feldspar ores by new flotation collectors. Miner Eng 11(12):1201–1208
Jada A, Akbour RA, Douch J (2006) Surface charge and adsorption from water onto quartz sand of humic acid. Chemosphere 64(8):1287–1295
Liu W, Liu W, Wei D, Li M, Zhao Q, Xu S (2017) Synthesis of N,N-Bis(2-hydroxypropyl)laurylamine and its flotation on quartz. Chem Eng J 309:63–69
Zhou Y, He C, Yang X (2008) Water contents and deformation mechanism in ductile shear zone of middle crust along the Red River fault in southwestern China. Sci China Ser D Earth Sci 51(10):1411–1425
Jin J, Gao H, Chen X, Peng Y (2016) The separation of kyanite from quartz by flotation at acidic pH. Miner Eng 92:221–228
Wang YH, Moitreyee MR, Kumar R, Shen L, Zeng KY, Chai JW, Pan JS (2004) A comparative study of low dielectric constant barrier layer, etch stop and hardmask films of hydrogenated amorphous Si-(C, O, N). Thin Solid Films 460(1–2):211–216
Wang L, Sun W, Hu Y-H, Xu L-H (2014) Adsorption mechanism of mixed anionic/cationic collectors in muscovite-quartz flotation system. Miner Eng 64:44–50
Luo B, Zhu Y, Sun C, Li Y, Han Y (2018) The flotation behavior and adsorption mechanisms of 2-((2-(decyloxy)ethyl)amino)lauric acid on quartz surface. Miner Eng 117:121–126
Filippov L, Severov V, Filippova I (2013) Mechanism of starch adsorption on Fe-mg-Al-bearing amphiboles. Int J Miner Process 123:120–128
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No.51804294,No.51874272, and No.51474201); Science and Technology Major Project of Sichuan Province (2019YFSY0029); Anhui Provincial Natural Science Foundation (No. 1808085ME121); Key Laboratory of Photovoltaic and Energy Conservation Materials, Chinese Academy of Sciences (No.PECL2018QN002); CPSF-CAS Joint Foundation for Excellent Postdoctoral Fellows (No. 2016LH0017); CASHIPS Director’s Funds (Grant No. YYJJ201624), International Clean Energy Talent Program by China Scholarship Council.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, M., Ban, B., Li, J. et al. Flotation Behavior, Collector Adsorption Mechanism of Quartz and Feldspar-Quartz Systems Using PEA as a Novel Green Collector. Silicon 12, 327–338 (2020). https://doi.org/10.1007/s12633-019-00135-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-019-00135-3