Abstract
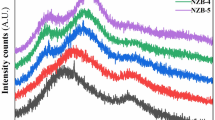
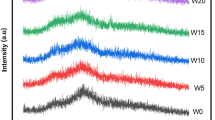
Infrared absorption spectroscopy measurements for a number of Na-borosilicate glasses containing AgBr and Cu2O were carried out in order to elucidate the structure of these glasses and the forms in which the transition metal ions exist in it. The raw materials used were of chemically pure grade and were finely pulverized. The temperature of the melting was 1,450 °C for 2 h and the samples were then annealed at 480 °C. The prepared glasses studied were then heat treated at 600 °C for 1 h and cooled inside the furnace. The absorption measurements in the Infrared region of the spectrum were recorded in range of (4,000–400) cm−1 using a Jacso FT-IR 300E Infrared spectrophotometer for all glasses studied. The experimental results obtained showed characteristic variations of the position and intensity of the absorption bands with the glass composition. The effect of the different transition metal oxides on the deformation of the groups present was investigated.
Similar content being viewed by others
References
Barker AS, Sievers AI (1975) J Mod Phys 47:1
Condrate AR Sr (1972) In: Pye LD, Stevens HJ, LaCourse WC (eds) Introduction to glass science. Plenum Publishing Corporation, New York, NY
Furukawa T, White WB (1980) 12th international congress on glass. Albuquerque, New Mexico
Efimov AM (1999) J Non-Cryst Solids 235:95
Kamitsos EI, Varsamis CPE, Vegeri A (2001) In: Proceeding of the international glass congress, Edinburgh, Scotland, Invited paper, I:234–246
Kamitsos EI (2003) J Phys Chem Glasses 44:79
Exarhos GI (1986) In: Walrafen GE, Revesz AG (eds) Structure and bonding in non-crystalline solids. Plenum, New York, NY, p 203
Bamford CR (1977) Colour generation and control in glass, Glass science & technology. Elsevier Scientific Publishing Company, Amsterdam
Khalil MM (2007) J Appl Phys A 86:505
El-Badry KhM, Afifi MS, Moustaffa FA, El-Sayedkh A (1984) Egypt J Phys 15(1):143
El-Badry KhM, Afifi MS, Moustaffa FA, Khalil EM (1983) CGCRI Bull (Indian) 30:11
Sharaf NA, Condrate AR Sr, Ahmed AA (1991) J Mater Lett 11(3):4
Rulmont A, Tarte P, Khorari S, Almou M (1996) Eur J Solid State Inorg Chem T 33:537
Khalil MI, ElBatal FH, Nada N, Desouky SA (2003) Indian J Pure Appl Phys 41:651
ElBatal FH, Nada N, Desouky SA, Khalil MI (2004) Indian J Pure Appl Phys 42:711
El-Egili (2003) J Phys B 325:340
Raluca CL, Ioan A (2007) J Non-Cryst Solids 353:2020–2024
Wan J, Cheng J, Lu P (2008) J Wuhan Univ Technol Mater Sci Ed 23(3)
Crozier D, Douglas RW (1965) Study of sodium silicate glasses in the Infrared by means of thin films. Phys Chem Glasses 6(6):240–245
El-Batal HA, Khalil EMA, Hamdy TM (2009) Ceram Int 35:1195
Azooz MA, AbouAiad THM, El-Batal FH, El-Tabii GT (2008) Indian J Pure Appl Phys 46:880
El-Batal FH, El Kheshen A (2008) Mater Chem Phys 110:352
Tarte P (1962) Spectrochim Acta 18:467
Condrate R (1972) Introduction to glass science. Plenum, New York, NY, p 101
El-Batal FH, Marzouk SY (2009) J Mater Sci 49:3061
Ferraro JR, Manghnani MH (1972) Infrared absorption spectra of sodium silicate glasses at high pressures. J Four Appl Phys 43(11):4595–4599
Husung DR, Doremus HR (1990) Rensselaer polytechnic institute. Troy, New York 12180–3590 J Mater Res 5:10
Efimov AM (2003) J Non-Cryst Solids 93:334
Scholze H (1966) Glass Ind 47(11):622
Dunken H, Doremus RH (1987) J Non-Cryst Solids 92:61
Navara G (2005) J Non-Cryst Solids 351:1796
Khalifa FA, El-Hadi ZA, Ghoneim NA, Moustaffa FA, Hassan NA (1987) CGCRI Bull 34:3
Navara G (2007) J Non-Cryst Solids 353:555
Sallem AA, Kellner R, Grasserbaver M (1994) Glass Technol 35:135
Moustaffa FA, Ghoneim NA, El-Hadi ZA, Ezz El-Din FM, Hasan NA (1985) CGCRI Bull 32:1
Efimov AM (1996) J Non-Cryst Solids 203:1
Kamitsos EI, Karakassided MA, Chryssikos GD (1986) Phys Chem Glasses 90:4528
Krogh-Moe J (1962) J Phys Chem Glasses 3:95
Krogh-Moe J (1965) J Phys Chem Glasses 6:46
Kamitsos EI, Patsis AP, Karakasides MA, Chryssikos GD (1990) J Non-Cryst Solids 126:52
El-Batal FH, Khalil MM, Nada N, Desouky SA (2003) Mater Chem Phys 82:375–387
Frey K, Funck E (1972) ZTS Naturfarh 27:101
Kamitsos EI, Karakassides MA (1989) J Phys Chem Glasses 30:229
Julien M, Balkanski M (1989) J Mater Sci Eng B3:307
Borrelli NF, Su GJ (1968) Infrared spectra of glass network formers and their interpretations. Mater Res Bull 3(2):181–192
Bell RJ, Bird ME, Dean P (1968) Proc Phys Soc Lond UK 1:299
Gaskell PH (1967) The vibrational spectra of silicates: Part I. Phys Chem Glasses 8(2):69–80
Bock J, Su GJ (1970) Interpretation of the infrared spectra of fused silica. J Four Am Ceram Soc 53(2):69–73
Blackman M (1957) Proc Phys Soc 70:827
Bienenstock A, Burley G (1963) Thermal expansion of silver iodide. J Four Phys Chem Solids 24:1271–1278
Weyl WA (1959) Coloured glasses. Monograph, Reprinted by Dawson’s of Pall Mall, London
Bates T (1962) In: Modern aspects of the vitreous state. Mackenzie JD (ed) Butterworths, London, 2:5
Zachariazen WH (1932) J Am Chem Soc 54:3841
Rao BV (1963) J Phys Chem Glasses 4:22
Rao BV (1964) J Am Ceram Soc 47:155
Yafaew NR, YablokovYuV (1962) Sov Phys Solids State 4:6
Morinaga K, Yoshida H, Takebe H (1994) J Am Ceram Soc 77(12):3113
Jen JS, Kalinowski MR (1979) J Non Cryst Solids
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saad, E.A., ElBatal, F.H., Fayad, A.M. et al. Infrared Absorption Spectra of Some Na-Borosilicate Glasses Containing AgBr and Cu2O (Photochromic Glasses) in Addition to One of Transition Metal Oxide. Silicon 3, 85–95 (2011). https://doi.org/10.1007/s12633-011-9081-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-011-9081-z