Abstract
Purpose
Brain death/death by neurologic criteria (BD/DNC) may be determined in many countries by a clinical examination that shows coma, brainstem areflexia, and apnea, provided the conditions causing reversible loss of brain function are excluded a priori. To date, accounts of recovery from BD/DNC in adults have been limited to noncompliance with guidelines.
Clinical features
We report the case of a 72-yr-old man with a combined primary infratentorial (hemorrhagic) and secondary global (anoxic) brain lesion in whom decompressive craniectomy of the posterior fossa and six-hour therapeutic hypothermia (33–34°C) followed by 8-hour rewarming to ≥ 36°C were conducted. Thirteen hours later, clinical findings of brain function loss were documented in addition to guideline-compliant exclusion of reversible causes (arterial hypotension, intoxication, depressant drug effects, relevant metabolic or endocrine disequilibrium, chronic hypercapnia, neuromuscular disorders, and administration of a muscle relaxant). Since a primary infratentorial brain lesion was present, German guidelines required further ancillary testing. Doppler ultrasonography revealed some preserved cerebral circulation, and BD/DNC was not diagnosed. Approximately 24 hr after rewarming to ≥ 36°C, the patient exhibited respiratory efforts. He continued with assisted respiration until final asystole/apnea, without regaining additional brain function other than mild signs of hemispasticity. Follow-up computed tomography showed partial herniation of the cerebellum through the craniectomy gap of the posterior fossa, alleviating caudal brain stem compression.
Conclusions
Therapeutic decompressive craniectomy of the posterior fossa may allow for delayed reversal of apnea. In these patients, proof of cerebral circulatory arrest should be mandatory for diagnosing BD/DNC.
Résumé
Objectif
Dans de nombreux pays, la mort cérébrale / décès déterminé par des critères neurologiques (MC / DDN) peut être déterminée par un examen clinique qui montre le coma, l’aréflexie du tronc cérébral et l’apnée, sous réserve que les conditions causant une perte réversible de la fonction cérébrale soient exclues a priori. À ce jour, les comptes rendus décrivant un rétablissement après une MC / DDN chez les adultes ont été limités en raison d’un non-respect des lignes directrices.
Caractéristiques cliniques
Nous rapportons le cas d’un homme de 72 ans atteint d’une lésion cérébrale sous-tentorielle primaire (hémorragique) et secondaire globale (anoxique) chez qui une craniectomie décompressive de la fosse postérieure et une hypothermie thérapeutique de six heures (33-34 °C), suivie d’un réchauffement de 8 heures à ≥ 36 °C, ont été réalisés. Treize heures plus tard, les résultats cliniques de la perte de la fonction cérébrale ont été documentés, en plus de l’exclusion conforme aux lignes directrices des causes réversibles (hypotension artérielle, intoxication, effets des médicaments dépresseurs, déséquilibre métabolique ou endocrinien pertinent, hypercapnie chronique, troubles neuromusculaires et administration d’un relaxant musculaire). Étant donné qu’une lésion cérébrale sous-tentorielle primaire était présente, les directives allemandes exigeaient la réalisation d’autres tests auxiliaires. L’échographie Doppler a révélé la préservation d’une certaine circulation cérébrale, et la MC / DDN n’a pas été diagnostiquée. Environ 24 heures après le réchauffement du patient à ≥ 36 °C, le patient a manifesté des efforts respiratoires. Il a continué à respirer avec assistance jusqu’à l’asystole / l’apnée finale, sans retrouver de fonction cérébrale supplémentaire autre que de légers signes d’hémispasticité. La tomodensitométrie de suivi a montré une hernie partielle du cervelet à travers l’espace de craniectomie de la fosse postérieure, soulageant la compression caudale du tronc cérébral.
Conclusion
La craniectomie décompressive thérapeutique de la fosse postérieure peut permettre une inversion retardée de l’apnée. Chez ces patients, la preuve d’un arrêt circulatoire cérébral devrait être obligatoire pour diagnostiquer une MC / DDN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The World Brain Death Project summarized minimum clinical standards for determination of brain death/death by neurologic criteria (BD/DNC),1 adopting primarily the American Academy of Neurology guidelines which require one clinical investigation and usually no ancillary testing irrespective of the type of brain lesion.2 In several countries, stricter standards apply, requiring ancillary testing and/or a second clinical investigation after a defined waiting period.1, 3 Still, contemporary studies involving > 2,600 patients with a second complete clinical investigation detected no reversal of findings.3,4,5 We report a case with apnea reversal and propose a red flag requiring proof of cerebral circulatory arrest.
Case report
This 72-yr-old retired seaman had a history of arterial hypertension and coronary heart disease. He was fully mobile, had stopped smoking 48 years ago, and took acetylsalicylic acid 100 mg, ramipril 5 mg, and simvastatin 20 mg daily.
Day 1
While bicycling, he experienced a sudden headache and dyspnea (15:30), dismounted the bicycle, and gradually lost consciousness. The Emergency Medical Service (EMS) was called (15:42). The EMS’ on-site emergency physician found the patient apneic, asystolic, and deeply comatose (15:53). Guideline-compliant cardiopulmonary resuscitation with endotracheal intubation/ventilation (16:00) and a total of 4,000 µg iv epinephrine returned spontaneous circulation with a stable sinus rhythm (16:05). Because of suspected acute myocardial ischemia, heparin 5,000 IU and acetylsalicylic acid 250 mg were administered intravenously.
The patient was taken to the local emergency room. On arrival (17:05), he remained deeply comatose without sedatives administered, with normal isochoric pupils slowly reacting to light stimuli. Fast-track ultrasonography showed normal cardiac function. To prevent post-anoxic encephalopathy, therapeutic hypothermia targeting 33–34°C was initiated using a core cooling device (17:25). Norepinephrine was administered via continuous iv infusion, along with Ringer’s solution for volume substitution. Cerebral computed tomography (CCT) revealed cerebellar and concomitant intraventricular hemorrhage, along with beginning occlusive hydrocephalus (17:45). He was urgently transferred to our center for decompressive craniectomy.
On arrival (19:22), his pupils were mydriatic bilaterally, with no response to light stimuli. Propofol was administered via continuous iv infusion at 4 mg·kg−1·hr−1 (67 µg·kg−1·min−1) between 19:50 and 23:05 (cumulative dose, 13 mg·kg−1). Sufentanil was given as analgesic comedication in three boluses (0.25, 0.125, and 0.125 µg·kg−1 at 19:55, 20:15, and 21:25, respectively). Occipital bore-hole trepanation via Frazier’s point (20:10) and dura mater incision revealed high intracranial pressure (ICP). Following external ventricular drainage, suboccipital craniectomy with cerebellar hematoma evacuation was performed, and, again, increased ICP with protruding cerebellar tissue was observed after dura mater incision (20:45). A postoperative CCT (22:01) showed transtentorial and transforaminal herniation (Electronic Supplementary Material [ESM], eFig. 1) and his pupils remained dilated and nonreactive bilaterally. Interdisciplinary agreement on a palliative therapy regimen was reached. Given the potential for organ donation, continued intensive care aimed at organ protection and gradual rewarming was initiated (23:30).
Day 2
The patient’s body core temperature rose above 36°C (07:20), but later temporarily dropped to < 36°C (20:00–21:00) (Fig. 1). Coma, apnea (i.e., no active triggering of the respirator), loss of brainstem reflexes, and loss of any reaction to pain stimuli persisted and the BD/DNC protocol was initiated (20:15).3 The brain lesion was classified as combined primary infratentorial and secondary (anoxic). The following potential confounders were excluded: arterial hypotension, hypothermia, intoxication, relevant metabolic or endocrine disequilibrium, chronic hypercapnia, neuromuscular disorders, and administration of a muscle relaxant. The plasma level of propofol was noted to be subtherapeutic (< 0.2 µg·mL−1) (08:00) and the plasma level of sufentanil was estimated in the lower therapeutic or subtherapeutic range (see ESM eAppendix case description). To further exclude a residual sufentanil effect, 0.005 mg·kg−1 iv naloxone was administered. Two physicians independently assessed the patient (20:25) and found that he was comatose, without any reaction to painful stimuli in the face and all four limbs. Pupillary light response, cephalo-ocular, corneal, gag, and cough reflexes were absent.1,2,–3, 6, 7 An apnea test was performed with positive airway pressure. During an increase in the arterial partial pressure of carbon dioxide to > 60 mm Hg (8 kPa) and subsequent disconnection from the respirator for another three minutes (ESM eTable), the patient showed no effort to breathe. Finally, ancillary testing was mandatory because of the primary infratentorial brain lesion.3, 8 Doppler ultrasonography (21:25) revealed some cerebral circulation (ESM eFig. 2). Therefore, BD/DNC was not diagnosed.
Diagram showing the timeline of core temperature and clinical events. Body core temperatures measured with a urinary bladder thermometer are displayed (•). The duration of medical measures/clinical events is shown by colored bands: targeted temperature management using a core cooling device (dark blue), administration of a cumulative dose of 13 mg·kg−1 propofol iv (light green), administration of a cumulative dose of 0.50 µg·kg−1 sufentanil iv (dark green), gradual rewarming to normothermia ≥ 36°C (light blue), clinical investigation and Doppler ultrasonography according to the guideline for diagnosing brain death (red), assisted spontaneous breathing (orange), and terminal asystole/apnea (black) (Color figure online).
Day 3
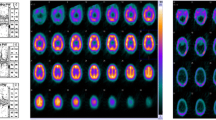
Computed tomography angiography (01:30) replicated the Doppler findings (Fig. 2). At 07:00, an effort by the patient to breathe was documented on monitoring. The respirator was switched to assisted spontaneous breathing mode. Neurologic investigation (11:50) showed persisting deep coma, bilaterally dilated, nonreactive pupils, and absent cephalo-ocular, corneal, gag, and cough reflexes. Slightly increased muscle tone of right-sided extremities with a positive Trömner reflex and right-ankle clonus was noted. Mild sensory stimuli-evoked invariable movements (head turning, right-sided shoulder-arm movements) were regarded as spinal automatisms.
Cerebral computed tomography (CCT) findings 33.5 hr after cardiopulmonary resuscitation. A–C, F) Nonenhanced CCT images showing partial herniation of the cerebellum through the craniectomy gap of the posterior fossa (C: white arrow; F: black arrow), alleviating brain stem compression by some amount. D, E) Contrast-enhanced CT angiography, obtained with a standardized protocol for proof of cerebral circulatory arrest, contradicted arrest of cerebral circulation, with opacification of the left middle and anterior cerebral arteries (D), weak opacification of the right middle and anterior cerebral arteries (D), and weak opacification of the basilar artery (E; arrow) by the contrast medium.
Day 4
Without regaining any clinically detectable brain function apart from respiratory drive and mild hemispasticity, the patient died of spontaneous asystole (21:23).
Discussion
The present case is unusual since a clinical sign of brain function loss reversed despite the exclusion of reversible causes provided in guidelines. Notably, posterior fossa craniectomy was followed by reversible apnea. Various national and international guidelines provide differing requirements and precluded the diagnosis of BD/DNC in this case; however, these criteria might fail in similar cases.
The American Academy of Neurology guidelines would not have allowed a diagnosis of BD/DNC due to the temperature criterion (≥ 36°C) but could possibly fail if the patient were half a degree warmer (Table 1).2 Twelve hours after the core temperature was > 36°C, the temperature fell slightly below 36°C just in the hour when the clinical investigations were performed. The guidelines of many European countries permit a lower core temperature (UK: > 34°C; elsewhere: > 35°C).1, 3, 7 Still, the application of therapeutic hypothermia, though shorter (six hours) than usual (> 24 hr), in combination with the administration of central nervous system (CNS)-depressant drugs in our case and their potential influence on the clinical findings necessitate special consideration.1,2,–3, 6, 7 The assessment was performed 13–16 hr after rewarming (depending on the temperature goal); had it waited for 24 hr after rewarming to ≥ 36°C, as recently recommended,1 the patient would just not have fulfilled the criteria. This, however, may not exclude a later apnea reversal in similar cases. The American Academy of Neurology and several European guidelines permit the determination of subtherapeutic drug plasma levels as an alternative to calculating the clearance based on half-lives of all applied CNS depressants, or, if appropriate, the administration of antidotes (naloxone or flumazenil) to exclude the presence of a CNS-depressant drug effect.2, 3, 6, 7 Although the plasma level of sufentanil was not measured in our patient, an effect was considered unlikely since the cumulative dose (administered within a short time window) was relatively low and naloxone was administered. The propofol plasma concentration was also determined to be subtherapeutic. Our case differs from earlier reported cases with neurologic recovery following premature declaration of BD/DNC after a cardiac arrest.9, 10 In these, duration of therapeutic hypothermia was longer (≥ 24 hr), and, importantly, CNS depressants (midazolam/phenobarbital in a ten-month-old child; propofol/fentanyl in a 55-yr-old man) were continuously administered over more than 24 hr.
The combination of anoxic brain injury, which often affects the brainstem less severely than the forebrain,11 and increased ICP resulting in transforaminal herniation of the caudal medulla, together with their partial reversibility, may explain the reversal of apnea. Posterior fossa decompression has been associated with restitution of brainstem functions, especially the respiratory drive.12, 13 Harvey Cushing was the first to document apnea reversal with decreasing pressure in the posterior fossa, which he attributed to improved brainstem perfusion.12 In the present case, delayed herniation of the cerebellum through the craniectomy gap (Fig. 2) may have caused relief to the medulla and, thereby, the return of spontaneous respiration. Of note, also bilateral supratentorial decompressive craniectomy may reverse the clinical signs of BD/DNC. Such a case recently prompted the addition of therapeutic decompressive craniectomy as a red flag, indicating the potential need for ancillary testing, in the UK.7
Accepted ancillary tests in patients with primary infratentorial brain lesions are electroencephalography (EEG), provided the exclusion of confounders, and imaging modalities proving cerebral circulatory arrest.1, 3, 8 Nevertheless, in our case, only some lower brainstem function but no other clinically detectable brain function had recovered. Considering the severe post-anoxic brain edema, an EEG, not recorded here, may well have shown electrocortical inactivity (ESM eFig. 1). The missing EEG notwithstanding, surface EEG can neither confirm nor exclude lower brainstem function.1, 8 In patients with intact skull and a primary infratentorial brain lesion, the loss of any motor response, brainstem reflexes, and breathing drive may be, but often is not the consequence of the brainstem lesion per se;8, 14 rather, the accompanying aqueduct occlusion results in supratentorial hydrocephalus, or there is a combined infratentorial and supratentorial brain lesion, both resulting in craniocaudal brain compression. In such a situation, the clinical findings of BD/DNC in combination with the EEG finding of electrocortical inactivity reliably show the irreversible loss of brain function.8 Nevertheless, if a skull defect prevents sustained compression of the lower brainstem, a partial recovery may occur. Even though the course in our patient was fatal, and coma persisted, the recovery of respiratory drive contradicts the widely accepted concept of BD/DNC.1,2,–3, 6,7,–8 Therefore, ancillary testing with EEG appears to be improper in patients with suboccipital decompressive craniectomy. Instead, the demonstration of complete cerebral circulatory arrest, along with the clinical findings of BD/DNC, reliably excludes recovery of brain function.8, 15 Cerebral circulatory arrest is the consequence of a supercritical increase of ICP. Preventing a posterior fossa ICP increase by suboccipital craniectomy allows blood supply to the lower brainstem, which is a prerequisite of apnea reversal.
We conclude that therapeutic decompression of the posterior fossa is a condition requiring proof of cerebral circulatory arrest to confirm BD/DNC.
References
Greer DM, Shemie SD, Lewis A, et al. Determination of brain death/death by neurologic criteria: The World Brain Death Project. JAMA 2020; 324: 1078–97.
Wijdicks EF, Varelas PN, Gronseth GS, Greer DM; American Academy of Neurology. Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010; 74: 1911–8.
Hoffmann O, Masuhr F. Use of observational periods or ancillary tests in the determination of brain death in Germany. Eur Neurol 2015; 74: 11–7.
Lustbader D, O'Hara D, Wijdicks EF, et al. Second brain death examination may negatively affect organ donation. Neurology 2011; 76: 119–24.
Varelas PN, Rehman M, Mehta C, et al. Comparison of 1 vs 2 brain death examinations on time to death pronouncement and organ donation: a 12-year single center experience. Neurology 2021; 96: e1453–61.
Wijdicks EF. Brain death guidelines explained. Semin Neurol 2015; 35: 105–15.
The Faculty of Intensive Care Medicine. Diagnosing Death using Neurological Criteria. Available from URL: https://www.ficm.ac.uk/diagnosing-death-using-neurological-criteria (accessed January 2022).
Walter U, Fernández-Torre JL, Kirschstein T, Laureys S. When is "brainstem death" brain death? The case for ancillary testing in primary infratentorial brain lesion. Clin Neurophysiol 2018; 129: 2451–65.
Joffe AR, Kolski H, Duff J, deCaen AR. A 10-month-old infant with reversible findings of brain death. Pediatr Neurol 2009; 41: 378–82.
Webb AC, Samuels OB. Reversible brain death after cardiopulmonary arrest and induced hypothermia. Crit Care Med 2011; 39: 1538–42.
Ryoo SM, Jeon SB, Sohn CH, et al. Predicting outcome with diffusion-weighted imaging in cardiac arrest patients receiving hypothermia therapy: multicenter retrospective cohort study. Crit Care Med 2015; 43: 2370–7.
Cushing H. Some experimental and clinical observations concerning states of increased intracranial tension. Am J Med Sci 1902; 124: 375–400.
Ammar A, Awada A, al-Luwami I. Reversibility of severe brain stem dysfunction in children. Acta Neurochir (Wien) 1993; 124: 86–91.
Manara A, Varelas P, Wijdicks EF. Brain death in patients with "isolated" brainstem lesions: a case against controversy. J Neurosurg Anesthesiol 2019; 31: 171–3.
Plourde G, Briard JN, Shemie SD, Shankar JJ, Chassé M. Flow is not perfusion, and perfusion is not function: ancillary testing for the diagnosis of brain death. Can J Anesth 2021; 68: 953–61.
Author contributions
Uwe Walter contributed to case study design, acquisition of data, analysis and interpretation of data, writing the manuscript, and critical revision. Maximilian Eggert, Udo Walther, Jürgen Kreienmeyer, Christian Henker, Hanka Arndt, Daniel Cantré, and Amelie Zitzmann contributed to acquisition of data, analysis and interpretation of data, and critically revising the manuscript for important intellectual content.
Acknowledgements
The authors thank the relatives of the deceased patient for agreeing to this case report. The study was approved by the ethics committee at the Rostock University Medical Center (identifier: A2021-0090).
Funding
The authors received no funding supporting the submitted work. Uwe Walter is an appointed member of the Permanent Working Committee on the “Guideline for the determination of irreversible loss of brain function” of the Scientific Advisory Board of the German Medical Association (Bundesärztekammer). Maximilian Eggert, Udo Walther, Jürgen Kreienmeyer, Christian Henker, Hanka Arndt, Daniel Cantré, and Amelie Zitzmann do not report any commercial or noncommercial affiliations that are or may be perceived to be a conflict of interest with the work. Uwe Walter has received speaker honoraria and travel funds from Bayer Vital, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Merz Pharma and Pfizer, and a research grant from Merz Pharma. He has received royalties from Thieme and Elsevier Press. He serves as editor of the European Journal of Ultrasound. Christian Henker has received speaker honoraria from Corza Medical. Maximilian Eggert, Udo Walther, Jürgen Kreienmeyer, Hanka Arndt, Daniel Cantré, and Amelie Zitzmann do not report any conflicting interests.
Editorial responsibility
This submission was handled by Dr. Alana M. Flexman, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is accompanied by an editorial. Please see Can J Anesth 2022; this issue.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Walter, U., Eggert, M., Walther, U. et al. A red flag for diagnosing brain death: decompressive craniectomy of the posterior fossa. Can J Anesth/J Can Anesth 69, 900–906 (2022). https://doi.org/10.1007/s12630-022-02265-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02265-6