Abstract
Purpose
The erector spinae plane (ESP) block is an interfascial analgesic technique first described as an alternative for pain control at the thoracic level. The objective of this observational study was to determine the anatomical spread of dye following a T7 ESP block in a cadaveric model.
Methods
An ultrasound-guided ESP block was performed in four fresh human cadavers using an in-plane approach with a linear probe in a longitudinal orientation and a puncture in a craniocaudal direction. Twenty millilitres of an iodinated contrast/methylene blue solution was injected deep to the erector spinae muscle at the distal end of the T7 transverse process bilaterally in two of the specimens, and unilaterally in the other two (six ESP blocks in total). Subsequently, the specimens were subjected to a multi-slice computed tomography (CT) scan with three-dimensional reconstruction. Two of the specimens were dissected to evaluate the distribution of the contrast solution, and a sectional study was performed in the other two.
Results
In the six samples, evaluated by CT scan and anatomical dissection, a craniocaudal spread of the dye was observed in the dorsal region from T1–T11 with lateral extension towards the costotransverse region. No diffusion of contrast solution or dye to the anterior region (paravertebral space) was observed by CT scan or dissection.
Conclusions
The results suggest that the ESP block reaches a wide range of the posterior rami of spinal nerves without diffusion into the paravertebral space or involvement of the anterior rami.
Résumé
Objectif
Le bloc du plan des muscles érecteurs du rachis (ESP) est une technique analgésique interfasciale qui avait d’abord été décrite comme une alternative pour contrôler la douleur au niveau thoracique. L’objectif de cette étude observationnelle était de déterminer la propagation anatomique d’un colorant après la réalisation d’un bloc ESP au niveau T7 dans un modèle cadavérique.
Méthode
Un bloc ESP a été réalisé sous échoguidage sur quatre cadavres humains frais en utilisant une approche dans le plan avec une sonde linéaire en orientation longitudinale et une ponction en direction cranio-caudale. Vingt millilitres d’une solution de contraste iodée / bleu de méthylène ont été injectés postérieurement aux muscles érecteurs du rachis à l’extrémité distale de l’apophyse transverse T7, bilatéralement dans deux des spécimens et unilatéralement dans les deux autres (soit six blocs ESP au total). Par la suite, les spécimens ont été soumis à une tomodensitométrie multicoupe avec reconstruction en 3D. Deux des spécimens ont été disséqués afin d’évaluer la distribution de la solution de contraste, et une étude sectionnelle a été réalisée sur les deux autres spécimens.
Résultats
Dans les six échantillons évalués par tomodensitométrie et dissection anatomique, une propagation cranio-caudale du colorant a été observée dans la région dorsale de T1–T11 avec une extension latérale vers la région costo-transverse. La tomodensitométrie et la dissection n’ont révélé aucune propagation de la solution de contraste ou du colorant à la région antérieure (espace paravertébral).
Conclusion
Ces résultats suggèrent que le bloc ESP atteint de nombreux rameaux postérieurs des nerfs rachidiens sans diffusion dans l’espace paravertébral ou atteintes des rameaux antérieurs.
Similar content being viewed by others
The erector spinae plane (ESP) block is an analgesic technique initially described as an alternative for the management of chronic neuropathic pain at the thoracic level.1 Its technical simplicity, analgesic efficacy, and low probability of complications has motivated its use in the management of acute postoperative pain in thoracic,2,3,4 abdominal,5,6 breast,7,8,9 shoulder,10 and (recently) spinal surgery.11
This ultrasound-guided block is performed using an in-plane parasagittal approach at the dorsal level of the trunk with the transverse process as a reference point for the puncture site. This site is the origin and insertion point of multiple muscle tendons and a complex framework of anatomical structures that partly hampers a clear understanding of the ESP block’s exact mechanism of action. Nevertheless, there are multiple case series that show the block’s wide anesthetic extension in the craniocaudal and anteroposterior directions of the trunk.1,2,3,4,5,6,7,8,9,10,11,12 This suggests a probable anterior diffusion of the local anesthetic solution into the paravertebral space by penetration of the intertransverse connective tissue, resulting in a blockade of the dorsal and ventral rami of the spinal nerves.
The objective of the present observational study was to describe the anatomical distribution of an ESP block using radiocontrast dye mixture injected at the level of the T7 transverse process in cadavers to determine the craniocaudal extension and the anterior dissemination towards the paravertebral space and the involvement of the dorsal and ventral rami of spinal nerves.
Methods
The present study was an anatomical descriptive study in which four human cadavers were used. The study complied with the institutional and ethical standards of the School of Medicine of the University of Barcelona (approved 21 June, 2017). The fresh anatomical specimens (trunk) corresponded to human donor bodies of advanced age without history of neoplastic pathology, impairment of the spine, or prior vertebral surgeries.
The ESP block was performed with each cadaver in the prone position using ultrasound guidance (M Turbo, Sonosite Inc., Bothell, WA, USA) with a 6–13-MHz high-frequency linear probe placed in longitudinal position (parasagittal section at the level of the transverse process), locating the tip of the T7 transverse process and, superficial to this tip, the deep plane of the erector spinae muscle. The T7 level was identified by counting from the first rib and descending caudally to the seventh rib. This rib was followed medially to identify the corresponding transverse process, which was marked on the cutaneous surface. The location was confirmed using the inverse process counting cranially from the 12th rib. Under ultrasound guidance, an in-plane puncture was performed in a craniocaudal direction with a 22G needle (Stimuplex D 50 mm needle, Braun, Melsungen, Germany) with the needle advancing until it contacted the lateral edge of the transverse process. At this point, 20 mL of a radiocontrast dye mixture (10 mL of iodexol 240 mg·mL−1, 1 mL of methylene blue, and 9 mL saline solution) was injected while observing the separation of the sub-erector spinae interfascial plane. The same procedure was performed in the subsequent three specimens, always taking the T7 transverse process as the reference point. The puncture was bilateral in two of the anatomical subjects and unilateral in the other two.
After the ESP block was completed, the anatomical samples were transferred to the radiology room for imaging by multi-slice computed tomography (CT) scan (Somaton Sesation 64, Siemens Medical Systems, Erlangen, Germany). A scout view of 512 mm was obtained. The images were obtained with a rotation time of 0.5 sec, slice collimation of 0.6 mm, 120 kV, and 90 mA effective current. Axial, coronal, and sagittal reconstructions were performed with 3 mm sections. The time from the completion of the block to the completion of the radiologic study was between one and two hours. The images were evaluated by a radiologist and then reconstructed into a three-dimensional version using open source software (Osirix, Pixmeo Switzerland) to observe the spread of the radiocontrast. Subsequently, two of the anatomical specimens were dissected from the spinous process in the direction of the transverse process and ribs to visually evaluate the location of the methylene blue in the vicinity of the erector spinae muscles. After the dye was identified, the intercostal space was accessed and the intercostal nerve was identified. The costotransverse and the intertransverse ligaments, levatores costarum longi, and rotatoris muscles were removed to evaluate the staining of the nerve roots in the paravertebral space.
The other two anatomical specimens were frozen at −20°C for a minimum period of 48 hr. Subsequently, 2 cm-thick sagittal or axial slices were performed with the objective of investigating any passage of the dye contrast from the erector spinae muscle through the intertransverse zone and into the paravertebral space and the corresponding intervertebral foramen.
Results
The multi-slice CT and volumetric reconstruction showed a wide craniocaudal distribution with variable extension from T1–L3 and lateral extension towards the costotransverse region, affecting the spinalis, longissimus, and iliocostalis muscles. The radiocontrast did not spread to the paravertebral or epidural space. We did not detect iodinated contrast solution close to the intervertebral foramina, at any level of the thoracic spine (Figs 1 and 2 and video, available as Electronic Supplementary Material).
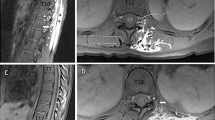
a Coronal computed tomography scan slices from the anterior (A) to the posterior (D) thoracic wall after the erector spinae plane block at the T7 level. Note the wide longitudinal distribution (C and D) following the erector spinae muscle. Nevertheless, no contrast is observed at the paravertebral level (A and B) and video, available as Electronic Supplementary Material. Fig. 1b Axial computed tomography slices after the erector spinae plane block at the T7 level (B). Note the dissemination of the contrast solution posterior to the transverse process in the depth of the erector spinae muscle and costotransverse joint without diffusion to the paravertebral space. Diffusion is also observed at other thoracic vertebral levels (T8, T10, and T12) (A) and at the intertransverse level of T7 and T8 (C) with the same pattern of spread
a A three-dimensional computed tomography reconstruction after the erector spinae plane block at the T7 level (white arrow). Note the extensive longitudinal diffusion along the erector spinae muscle, from its innermost component, the spinalis muscle, towards the outer component, the fascicles of the iliocostalis muscle. Fig. 2b A three-dimensional computed tomography reconstruction after the erector spinae plane block at the T7 level (white arrow). Note the extensive longitudinal diffusion along the erector spinae muscle (A) without anterior passage of the contrast (B). Note the perfect visualization of all the intervertebral foramina and the contrast posterior to the ribs (B)
The dorsal branch of the spinal nerve crosses the intertransverse space towards the erector spinae muscle, while the anterior branch of the spinal nerve remains in the space between the two ribs. We did not observe any diffusion of methylene blue anterior to the transverse process. The anatomical dissection of the two cadavers did not show staining of the anterior branch of the spinal nerve, as shown in Fig. 3. The sectional study of the other two samples corroborated with the findings of the CT scan study and the dissections. No methylene blue was observed in the area where the anterior branch of the spinal nerve is located (Fig. 4: sagittal sections). The Table qualitatively summarizes the diffusion of the methylene blue in each of the anatomical samples.
Sagittal slices of the anatomical specimen through the midline (A), through the laminar line (B), through the transverse processes (C), and at the level of the ribs (D). Note the presence of contrast solution (methylene blue) at the level of the iliospinalis (section B), longissimus (section C), and iliocostalis (section D). At no time does the dye exceed the posterior limit of the bony structures of the spine and trunk
Discussion
When the ESP block technique and its broad analgesic effect for the treatment of neuropathic pain in the thoracic wall was first described,1 the mechanism of action was hypothesized to be the penetration of the local anesthetic through the costotransverse ligament to reach the vicinity of the intervertebral foramina, with involvement of both the dorsal and ventral rami of spinal nerves, as shown by the distribution of cutaneous dermatomes.1,10
Nevertheless, this proposed mechanism was not confirmed in the present study, despite following the technical recommendations of the block and maintaining the same volume of contrast/dye administered. In the anatomical and radiologic aspects of this current investigation, a wide craniocaudal distribution of the contrast was observed at the level of the laminae (retrolaminar), the posterior region of the transverse process, and the depth of the erector spinae muscle, with a tendency towards lateral extension into the costotransverse region. Nevertheless, it has not been possible to show its dissemination into the paravertebral space, which suggests a wide block of the dorsal ramus without affecting the ventral ramus of the spinal nerves. Therefore, although the analgesic clinical effect thus far documented is not discussed, based on the anatomical study conducted, it cannot be concluded that the analgesic and anesthetic mechanism is equivalent to that of the paravertebral block.
Similar findings have recently been published by Ivanusic et al.13 in an anatomical study investigating the mechanism of action of an ESP block. In their study, a wide cephalic and lateral distribution of the contrast is described after 20 injections at the level of the T5 transverse process, showing in detail by dissection, the frequent staining of the dorsal branch after it exits from the costotransverse foramen, but without involving the anterior branch of the spinal nerve. Nevertheless, they found dye in one injection (5%) on the ventral ramus, and in two of the injections (10%), the dye tracked posteriorly through the costotransverse foramen to reach the dorsal root ganglia.
These results differ from those of Adhikary et al.14 who used magnetic resonance imaging (MRI) to assess the diffusion of contrast after performing ESP blocks and retrolaminar blocks in a cadaveric study. In that study, radiologic slices clearly showed a paravertebral diffusion that also involved the epidural space. Similar findings were observed in the clinical report of Schwartzmann et al.15 after ESP puncture at the T10 level in a patient with a history of chronic abdominal pain. In both studies, spinal nerve involvement occurred in several vertebral segments.
Although these studies are in contrast with our findings, it should be considered that MRI offers a higher spatial resolution and definition of soft tissues, superior to the CT scan used in our study. Moreover, the physico-chemical characteristics of the CT contrast compared with the MRI contrast may result in a different spread in soft tissues.16 Accordingly, it is prudent to consider the different factors that could influence the results in the studies done thus far, such as the exact needle tip position, variable tissue echogenicity, and ultrasound-machine resolution, as well as the contrast properties used for detecting the injectate spread.
From an anatomical point of view, the pattern of spread of the contrast/dye between the living patient and the cadaver can be variable, which constitutes a limitation of the study in terms of its translation to clinical practice. The erector spinae muscle, along its craniosacral extension, is inside a fascial retinaculum (the thoracolumbar fascia), and the tonicity of the musculature, which is lacking in the cadaver, could exert a pressure effect, permitting greater extension of the contrast towards the anterior costotransverse region, reaching the paravertebral space. Nevertheless, this extension could not be observed in cadaver studies.
On the other hand, as proposed by Yang et al.,17 the disposition and composition of the ligaments neighbouring the costotransverse joint vary along the spinal column. The posterior costotransverse ligament is absent, or rudimentary, between T1 and T6, and it is very well developed at the T7–T10 levels,14,18 which could negatively influence the diffusion within the paravertebral space at these levels, as in our study.
From a pharmacologic point of view, the clinical effect of a fascial block depends to a great extent of the spread of the injected anesthetic solution; thus, the volume administered could have an important effect on the results. In an anatomical study conducted in pigs by Damjanovska et al.19 to evaluate the spread of different volumes (30 mL vs 20 mL) of local anesthetics into the paravertebral space after an ultrasound-guided retrolaminar injection, the highest volume administered showed a spread towards the paravertebral space in all of the specimens, whereas in the lower volume group, the anesthetic solution did not diffuse anteriorly in any of the subjects. These findings suggest that the paravertebral space is not a closed volume and that anatomical communications with neighbouring structures are possible and have still to be elucidated. Though this may seem obvious, we still need to take into account the limitations of animal studies concerning the possible variations with the human anatomy.
Nevertheless, we cannot rule out other anatomical mechanisms, independent of the diffusion to the paravertebral space, which could contribute to the analgesic efficacy of the ESP block observed in clinical practice. The thoracolumbar fascia, which is a complex structure of several layers that forms a girdle with a sustaining and stabilizing function, seems to contain a high density of nerves and sympathetic fibres.20,21 This anatomical feature could explain a kind of modulation of somatic pain reaching further than seen with the distribution of the injected dye. Furthermore, given the observed lateral spread of the contrast in the costotransverse region, there could be an involvement of the lateral cutaneous branches of the intercostal nerves, as already stated in other anatomical studies.13 Spread to different nerves (or rami) and modulation by sympathetic fibres could be evaluated by thermal quantitative sensory testing22 in clinical investigations.
In addition to the good clinical results of ESP blocks that have been reported as part of multimodal analgesia programs showing reduced opioid consumption,23 the ESP block has other advantages. These advantages include the ease of ultrasound identification of the structures used as a reference (even in obese patients), and the relatively low risk of adverse effects (such as pneumothorax and injury to vascular and nervous structures). Thus, one can conclude that the anatomical results coupled with some clinical outcomes24 challenge the concept that the injectate spreads to the anterior ramus of the spinal nerves, and similarly challenges its role as a simple and alternative approach of paravertebral block. Nevertheless, further clinical investigations are necessary to corroborate or compare with results obtained in cadavers.
References
Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med 2016; 41: 621-7.
Forero M, Rajarathinam M, Adhikary S, Chin KJ. Continuous erector spinae plane block for rescue analgesia in thoracotomy after epidural failure: a case report. A A Case Rep 2017; 8: 254-6.
Forero M, Rajarathinam M, Adhikary S, Chin KJ. Erector spinae plane (ESP) block in the management of post thoracotomy pain syndrome: a case series. Scand J Pain 2017; 17: 325-9.
Adhikary SD, Pruett A, Forero M, Thiruvenkatarajan V. Erector spinae plane block as an alternative to epidural analgesia for post-operative analgesia following video-assisted thoracoscopic surgery: a case study and a literature review on the spread of local anaesthetic in the erector spinae plane. Indian J Anaesth 2018; 62: 75-8.
Chin KJ, Adhikary S, Sarwani N, Forero M. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia 2017; 72: 452-60.
Chin KJ, Malhas L, Perlas A. The erector spinae plane block provides visceral abdominal analgesia in bariatric surgery: a report of 3 cases. Reg Anesth Pain Med 2017; 42: 372-6.
Veiga M, Costa D, Brazao I. Erector spinae plane block for radical mastectomy: a new indication? (Spanish). Rev Esp Anestesiol Reanim 2018; 65: 112-5.
Ohgoshi Y, Ikeda T, Kurahashi K. Continuous erector spinae plane block provides effective perioperative analgesia for breast reconstruction using tissue expanders: a report of two cases. J Clin Anesth 2018; 44: 1-2.
Tanaka N, Ueshima H, Otake H. Erector spinae plane block for combined lovectomy and radical mastectomys. J Clin Anesth 2018; 45: 27-8.
Forero M, Rajarathinam M, Adhikary SD, Chin KJ. Erector spinae plane block for the management of chronic shoulder pain: a case report. Can J Anesth 2018; 65: 288-93.
Melvin JP, Schrot RJ, Chu GM, Chin KJ. Low thoracic erector spinae plane block for perioperative analgesia in lumbosacral spine surgery: a case series. Can J Anesth 2018; 65: 1057-65.
El-Boghdadly K, Pawa A. The erector spinae plane block: plane and simple. Anaesthesia 2017; 72: 434-8.
Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med 2018; 43: 567-71.
Adhikary SD, Bernard S, Lopez H, Chin KJ. Erector Spinae plane block versus retrolaminar block: a magnetic resonance imaging and anatomical study. Reg Anesth Pain Med 2018; 43: 756-61.
Schwartzmann A, Peng P, Antunez M, Forero M. Mechanism of the erector spinae plane block: insights from a magnetic resonance imaging study. Can J Anesth 2018; 65: 1165-6.
Peters AM. Fundamentals of tracer kinetics for radiologists. Br J Radiol 1998; 71: 1116-29.
Yang HM, Choi YJ, Kwon HJ, O J, Cho TH, Kim SH. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia 2018; 73: 1244-50.
Ibrahim AF, Darwish HH. The costotransverse ligaments in human: a detailed anatomical study. Clin Anat 2005; 18: 340-5.
Damjanovska M, Pintaric TS, Cvetko E, Vlassakov K. The ultrasound-guided retrolaminar block: volume-dependent injectate distribution. J Pain Res 2018; 11: 293-9.
Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat 2012; 221: 507-36.
Tesarz J, Hoheisel U, Wiedenhofer B, Mense S. Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience 2011; 194: 302-8.
Sermeus LA, Hans GH, Schepens T, et al. Thermal quantitative sensory testing to assess the sensory effects of three local anesthetic solutions in a randomized trial of interscalene blockade for shoulder surgery. Can J Anesth 2016; 63: 46-55.
Tsui BC, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae (ESP) block: a pooled review of 242 cases. J Clin Anesth 2018; 53: 29-34.
Ueshima H, Otake H. Limitations of the erector spinae plane (ESP) block for radical mastectomy. J Clin Anesth 2018; 51: 97.
Competing interests
No external funding and no competing interests declared.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Adriana Aponte and Xavi Sala-Blanch conceived the study, participated in its design and coordination, performed the blocks and dissection, and wrote the manuscript. Alberto Prats-Galino participated in anatomy dissection and analysis. Joseph Masdeu helped in writing the manuscript. Luis A. Moreno helped in conceiving the study and performed the blocks. Luc A. Sermeus helped to conceive the study, helped to draft the manuscript, and performed a critical reading of the manuscript.
Disclosure of funding
No external funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is accompanied by an editorial. Please see Can J Anesth 2019; 66: this issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
VIDEO Image showing the spread of contrast solution and the calcifications in a series of axial computed tomography slices of the trunk after an erector spinae plane block. Supplementary material 1 (M4V 4828 kb)
Rights and permissions
About this article
Cite this article
Aponte, A., Sala-Blanch, X., Prats-Galino, A. et al. Anatomical evaluation of the extent of spread in the erector spinae plane block: a cadaveric study. Can J Anesth/J Can Anesth 66, 886–893 (2019). https://doi.org/10.1007/s12630-019-01399-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01399-4