Abstract
Introduction
Restrictive transfusion strategies have been advocated in critically ill patients. Nevertheless, considerable uncertainty exists regarding optimal transfusion thresholds in traumatic brain injury (TBI) patients because the injured brain is susceptible to hypoxemic damage. We aimed to identify the determinants of red blood cell (RBC) transfusion and the perceived optimal transfusion thresholds in adult patients with moderate-to-severe TBI.
Methods
We conducted an electronic, self-administered survey targeting critical care specialists and neurosurgeons from Canada, Australia, and the United Kingdom caring for TBI patients. The questionnaire was initially developed by a panel of experts using a structured process (domains/items generation and reduction). The questionnaire was validated for clinical sensibility, reliability, and content.
Results
The response rate was 28.7% (218/760). When presented with the hypothetical scenario of a young adult TBI patient, a wide range of transfusion practices was observed, with 47 (95% confidence interval [CI], 41 to 54)% favouring RBC transfusion at a hemoglobin level of ≤ 70 g·L–1 in the acute phase of care, while 73 (95% CI, 67 to 79)% would use this trigger in the plateau phase of care. Multiple trauma, neuro-monitoring data, hemorrhagic shock, and planned surgery were the main factors that influenced the need for transfusion. The lack of clinical evidence and guidelines was responsible for uncertainty regarding RBC transfusion strategies in this patient population.
Conclusion
In our survey about critically ill TBI patients, transfusion practice was found to be mainly influenced by the acuity of care, patient characteristics, and neuro-monitoring. Clinical equipoise regarding optimal transfusion strategy is believed to be mainly attributed to the lack of clear clinical evidence and guidelines. Appropriate randomized-controlled trials are required to determine the optimal transfusion strategies in TBI patients.
Résumé
Introduction
Le recours à des stratégies de transfusion restrictives a été préconisé chez les patients en état critique. Une incertitude considérable demeure toutefois quant aux seuils optimaux de transfusion pour les patients atteints de traumatisme cérébral (TCC) considérant que le cerveau lésé est susceptible de subir des lésions hypoxémiques. Nous avons tenté d’identifier les facteurs déterminants pour l’initiation d’une transfusion d’érythrocytes ansi que les seuils de transfusion considérés comme optimaux chez les patients adultes victimes de TCC modéré à grave.
Méthodologie
Nous avons développé un sondage électronique et auto-administré destiné aux intensivistes et aux neurochirurgiens canadiens, australiens et britanniques prenant soin de patients ayant subi un TCC. Le questionnaire a d’abord été élaboré par un groupe d’experts à l’aide d’un processus structuré (génération et réduction de domaines/items). Le questionnaire a ensuite été validé pour garantir sa sensibilité clinique, sa fiabilité et son contenu.
Résultats
Le taux de réponse était de 28,7% (218/760). Lorsqu’on a soumis aux répondants le cas hypothétique d’un jeune adulte ayant subi un TCC, nous avons observé un vaste éventail de pratiques transfusionnelles, 47% (intervalle de confiance [IC] 95%, 41 à 54%) des répondants étant en faveur d’une transfusion d’érythrocytes à partir d’un taux d’hémoglobine ≤ 70 g·L–1 dans la phase aiguë de soins, alors que 73% (IC 95%, 67 à 79%) utiliseraient ce seuil déclencheur dans la phase chronique des soins. Les traumatismes multiples, les données de monitorage neurologique, un choc hémorragique et une chirurgie programmée constituaient les principaux facteurs influençant le besoin perçu de transfusion. L’absence de données probantes cliniques et de recommandations était responsable de l’incertitude concernant les stratégies de transfusion d’érythrocytes chez cette population de patients.
Conclusion
Dans notre questionnaire portant sur les patients victimes de TCC en état critique, nous avons observé que les pratiques de transfusion étaient principalement influencées par l’acuité des soins, les caractéristiques des patients et le monitorage neurologique. L’équilibre clinique (equipoise) concernant la stratégie de transfusion optimale est probablement principalement attribuable à l’absence de données probantes cliniques claires ou de recommandations. Des études randomisées contrôlées sont nécessaires afin de déterminer quelles stratégies de transfusion seraient optimales pour les patients atteints de TCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Anemia and treatment with red blood cell (RBC) transfusion are common in critically ill patients following traumatic brain injury (TBI).1,2,3 Although anemia is associated with secondary brain injury,4 its independent association with unfavourable outcomes is uncertain.5,6,7 While RBC transfusion and the adoption of a more liberal transfusion strategy have been suggested to improve tissue oxygenation in the injured brain,8,9,10,11 several observational studies report deleterious effects of transfusion in this population.5,12,13,14
Large clinical trials have shown that restrictive transfusion strategies (higher threshold to transfuse) are as safe as liberal strategies (lower threshold) in critically ill pediatric and adult patients.15,16 Nevertheless, in some patient sub-groups, liberal transfusion policies may be associated with adverse outcomes.17 Of note, patients with TBI were underrepresented in these studies.18
While uncontrolled observational studies have reported an association between transfusion or transfusion volume and adverse neurological outcome in patients with TBI,5,9,12,13,19,20 few comparative studies have been conducted to clarify the effect of transfusion or anemia in this patient population,21 and very few have assessed the impact of transfusion on clinical outcome. In a trial of 200 TBI patients randomized to a restrictive or liberal RBC transfusion strategy, no difference in neurologic outcome was observed. Nevertheless, a higher incidence of thromboembolic events was observed in patients assigned to a liberal transfusion strategy and erythropoietin regimen.22
Considering the particular vulnerability of the injured brain to hypoxemia, transfusion strategies in TBI patients would seem to be of importance. While a previous survey showed that medical specialty can influence hemoglobin transfusion thresholds in TBI patients, the survey was not designed to identify factors influencing the transfusion strategy.23 Moreover, most recent guidelines concerned with the management of TBI patients do not provide recommendations regarding RBC transfusion.24
Given the uncertainty related to RBC transfusion in critically ill TBI patients, it is important to assess current transfusion strategies to help establish the contemporary standard of care. Therefore, we conducted an international survey of critical care specialists and neurosurgeons to evaluate their attitudes regarding RBC transfusion practices in critically ill TBI patients. We anticipated that these data would be useful to help orient future clinical trials.25
Methods
Study population
With approval from the Research Ethics Board of the CHU de Québec – Université Laval (REB# 2015-2309, 2015), we conducted a self-administered cross-sectional survey of Canadian, United Kingdom (UK), and Australian critical care specialists and neurosurgeons involved in the care of critically ill adult patients with TBI. Canadian level 1 and level 2 trauma centres were identified through provincial Ministries of Health and the Trauma Association of Canada. The major trauma centres in the UK were identified through the National Health Services, and Australian major trauma centres were identified through the National Trauma Research Institute. The institutional email address of each potential respondent was used to disseminate the survey.
Survey development
The development of the questionnaire was guided by recently published systematic reviews of current practices and determinants of RBC transfusion in critically ill TBI patients,21,26 and by a review of the relevant articles cited in these systematic reviews. Our methodologic plan followed current standards for health survey research.27,28 A list of domains and items pertaining to the study objectives was furnished by a panel of thirteen collaborators from Canada, the UK, and Australia, who were experts in critical care medicine, neurosurgery, hematology, and epidemiology. Domains that were identified comprised the main themes targeted by the questionnaire, while items comprised the sub-themes. Using a modified Delphi approach to arrive at a consensus,11 panel members identified the most relevant domains and items for the survey. In this process, a list of domains and items was first generated and then reduced to include those considered to be the most important and relevant to the study objectives.
Survey domains
Perceived importance of patient and injury-related factors when considering the need for RBC transfusions in TBI patients
Using a five-point Likert scale (very important, important, neither important or unimportant, of little importance, and unimportant), we examined the perceived importance of various indices including medical (e.g., brain physiology, injury severity, timing of injury, co-morbidities) and socio-demographic (e.g., age) factors that could influence the need for blood transfusion in TBI patients.
Factors influencing the perception of optimal hemoglobin threshold for transfusion in TBI patients
Starting with a hypothetical “baseline” clinical scenario, we assessed the perceived optimal hemoglobin threshold (from 60 to 110 g·L−1) to transfuse an adult patient with severe TBI. Subsequently, physiologic and clinical parameters of the scenario were modified and changes in perceived optimal transfusion thresholds were assessed.
Barriers and facilitators to the adoption of a restrictive or liberal transfusion strategy in TBI patients
We asked specific questions related to the perceived barriers and facilitators that influence the choice of a restrictive (hemoglobin threshold of 70 g·L−1) compared with a liberal (hemoglobin threshold 90 g·L−1) transfusion strategy. Questions included the presence of transfusion protocols, guidelines, and institutional practice.
Pre-survey testing
The survey was assessed for the validity of its content, ease of administration, and reliability using a three-step approach. A multidisciplinary group of clinicians, including neurosurgeons and critical care specialists from the CHU de Québec-Université Laval pilot-tested the survey instrument to evaluate its clarity, comprehensiveness, and redundancy. Second, we assessed the clinical sensibility of the survey instrument. The same group of clinicians evaluated the survey’s ease of use, question format, content validity, and redundant or missing items (Appendix). Finally, the reliability of the survey was evaluated. A group of four critical care medicine fellows and five neurosurgery residents completed the questionnaire on two different occasions. None of the clinicians who participated in the validation phase were involved in the Delphi approach and the development of the questionnaire. The survey questionnaire was subsequently revised according to the different phases of validation (see questionnaire eAppendix, available as Electronic Supplementary Material).
Survey administration
The questionnaire was disseminated using a web-based interface (LimeSurvey™) to potential respondents in Canada and the UK in November 2015 and to those in Australia in June 2016. Each potential respondent received a personalized email providing information regarding the rationale and objectives of the study. They were asked to confirm their involvement in the care of patients with TBI to ensure their eligibility. An electronic reminder was sent to those who did not respond two weeks after the initial invitation and was then repeated two weeks later.
Sample size
Based on a recent publication,29 we initially estimated the total number of potential respondents to be approximately 800, of which around 600 would meet eligibility criteria. With an anticipated response rate of 65% and a 90% rate of completing the questionnaire we expected to obtain about 350 participants. This sample size provided sufficient power to generate 95% confidence intervals (CI) of 3% width around a response selected by 50% of respondents (the proportion with the most conservative measure of dispersion). The measure of dispersion around our final observed sample size (n = 218) was 6%.
Statistical analyses
Questionnaires with more than 80% of questions answered were considered complete and used for statistical analysis.30 For the assessment of test-retest reliability, we used the weighted version of Gwet’s coefficient, AC2. This test is less vulnerable to the paradoxical very low coefficient agreements when the percent agreements (i.e., proportion of merged responses from a Likert scale) are in fact “high”.31 A Gwet’s AC2 score of 0.40 or greater was considered as acceptable, indicating at least “moderate” to “good” agreement. Scores ranged from 0.23 to 1.00. The four questions with a score lower than 0.4 were modified thereafter to ensure adequate reproducibility. We used descriptive statistics to present our results with proportions and 95% CI. A Chi-square test was used to compare proportions when necessary.
Results
Respondent characteristics
We identified 760 potential respondents in Canada, the UK, and Australia. We received 218 completed questionnaires meeting our inclusion requirements (28.7%, Fig. 1). Of respondents, 22% worked in the UK, 33% in Australia, and 45% in Canada. Since we could not send the survey to UK neurosurgeons using the same methodologic approach, we were unable, for confidentiality reasons, to obtain any data pertaining to dissemination or response rate for these respondents and so excluded their data to enable homogeneous reporting of results. The majority of respondents were intensive care physicians (88%) working in closed intensive care units (88%) that admit a mixture of medical/surgical patients (84%, Table). Almost 70% of physicians had been in practice for more than ten years. One-third of the respondents (34%) reported caring for over 100 moderate-to-severe TBI patients per year.
Perceived importance of patient- or injury-related factors when considering RBC transfusions in TBI patients
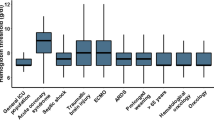
About half of the respondents reported that cerebral perfusion pressure (51 [95% CI, 44 to 57]%), cerebral blood flow (53 [46 to 60]%), brain tissue oxygenation (PbtO2, 52 [45 to 59]%), and intracranial pressure measurements (46 [40 to 53]%) were the main (important or very important) physiologic factors when considering the need for RBC transfusions (Fig. 2). About one-third (34 [28 to 41]%) of respondents reported that results from cerebral venous oxygen saturation monitoring and brain microdialysis were less important (unimportant or of little importance). A change in brain autoregulation was considered to be neither important nor unimportant by 43 (37 to 50)% of clinicians. Forty-five (39 to 51)% and 33 (27 to 39)% of clinicians felt that the type of lesions and severity of brain injury (moderate vs severe), respectively, was neither important nor unimportant when considering the need for transfusion. On the other hand, the need for surgery (65 [58 to 71]%), hemodynamic instability (77 [71 to 82]%), and the presence of multiple trauma (61 [54 to 67]%) were the main injury-related factors when considering the need for transfusion. Fifty-four (48 to 60)% of respondents reported that the acute or subacute phase of care was an important consideration in transfusion decisions. The presence of coronary arterial disease was considered to be an important or very important patient-related factor for 69 (62 to 74)% of respondents, compared with age (41 [35 to 48]%) or increased metabolic demands (39 [32 to 45]%).
Factors influencing the perception of optimal hemoglobin threshold for transfusion in patients with TBI
When presented with a “baseline” scenario of a stable 26-yr-old male with a severe blunt TBI (score of 6 on the Glasgow Coma Scale) admitted to the ICU with an intracranial pressure of 15 mmHg, 80 (74 to 85)% of respondents indicated that a hemoglobin of 80 g·L−1 or less was an appropriate transfusion threshold during the acute phase of care, and 47 (41 to 54)% favoured a threshold of ≤ 70 g·L−1 (Fig. 3). Only 6 (4 to 10)% reported that a higher transfusion trigger (100 g·L−1) was necessary. When considering the subacute or plateau phase of care in the same patient, 91 (87 to 94)% of physicians believed that a hemoglobin of 80 g·L−1 or less was an appropriate transfusion threshold, while 73 (67 to 79)% favoured a threshold of ≤ 70 g·L−1 compared with 2 (1 to 5)% who targeted a hemoglobin level of ≥ 100 g·L−1. When the baseline scenario was altered, the injury severity altered hemoglobin transfusion thresholds. For example, a threshold of ≥ 90 g·L−1 was considered to be more appropriate by about half of the respondents (51 [45 to 58]%) if the patient was in hemorrhagic shock or needed multiple surgeries (44 [37 to 50]%). Fifty-two (46 to 58)% of clinicians reported that an elevated intracranial pressure (25 mmHg) warranted a hemoglobin threshold of ≥ 80 g·L−1, and 18 (13 to 24)% chose a hemoglobin threshold of ≥ 100 g·L−1. The type of brain injury did not change perceptions of appropriate hemoglobin threshold levels. Older age was not associated with the need for a higher transfusion threshold, but a history of coronary arterial disease influenced transfusion decisions toward a hemoglobin threshold of ≥ 70 g·L−1 by 93 (89 to 96)% of clinicians.
Barriers and facilitators to the adoption of a restrictive or liberal transfusion strategy in TBI patients
The absence of clear guidelines and the current level of evidence were the strongest factors influencing the adoption of either transfusion strategy in TBI patients (Fig. 4). When asked about strategies to reduce the need for transfusion, limiting daily blood work and a closed system for arterial line (designed to reduce blood discard) were considered to be the most useful measures by most (86 [81 to 90]% and 66 [59 to 72]% of responders, respectively). Only 11 (7 to 15)% thought that erythropoietin administration was a useful modality compared with 45 (38 to 51)% for tranexamic acid. Approximately half of respondents could neither agree nor disagree when asked for their opinion regarding the superiority or inferiority of restrictive or liberal transfusion strategies with regard to clinical outcomes. Of the remainder, 29 (23 to 35)% believed that a restrictive strategy is associated with better clinical outcomes, 23 (18 to 29)% believed that both strategies would lead to similar outcomes, and 6 (4 to10)% favoured a liberal transfusion strategy.
Discussion
We surveyed intensive care physicians and neurosurgeons from Canada, the UK, and Australia regarding their attitudes and beliefs toward RBC transfusion strategies in critically ill patients with TBI. We observed variations in transfusion practices, mainly influenced by the timing and severity of injury. Most clinicians reported a more restrictive transfusion strategy in the management of moderate-to-severe TBI patients.
Our results are comparable with those of another survey performed in 2009 that assessed transfusion strategies in patients with TBI.23,32 In that survey, half of the respondents reported using a restrictive transfusion strategy, with neurosurgeons having a slight preference toward a more liberal transfusion strategy. Unlike our study, a subset of clinicians that were surveyed included those with an administrative role (e.g., ICU directors, heads of neurosurgery, and chiefs of trauma surgery)23 or members of a medical society,32 which may include clinicians not actively providing clinical care for these patients. These study design differences may help to explain why we did not observe a difference between medical specialties. We believe that our results reflect the contemporary transfusion practice of a more restrictive strategy in critically ill TBI patients.33 Notably, our results contrast with a recently published international survey regarding RBC transfusion in the acute brain-injured patient.31 In that study, most physicians reported adopting a restrictive hemoglobin threshold of ≤ 80 g·L−1 to trigger a transfusion in the critically ill population, but more than half of them would favour a threshold higher than 80 g·L−1 for patients with TBI. Of note, that survey targeted registered members of critical care societies and whose opinions may not reflect the broader community of critical care physicians.
Respondents to our survey did not use the same hemoglobin threshold for all patients. Instead, transfusion practice was influenced by specific clinical characteristics related to the severity of the injury, anticipated future blood loss, and reduced cerebral perfusion, where patients would be less likely to tolerate anemia. Similar concerns were observed in a North American survey of Canadians and US critical care physicians and vascular neurosurgeons on transfusion practice in patients with subarachnoid hemorrhage,33 which highlighted a persistent concern with the vulnerability of the injured brain to hypoxemic damage.
Neurologic monitoring (e.g., intracranial pressure and PbtO2 measurements) was considered to be an important influence on the hemoglobin threshold to transfuse a TBI patient. Changes in the cerebral perfusion pressure and cerebral blood flow affect brain oxygen delivery. Surrogate markers of oxygen delivery to the human brain following transfusion include the use of PbtO2 monitoring.8,10,11 Although not an actual standard of practice, PbtO2 monitoring was considered to be important by most clinicians. These results are surprising considering the lack of evidence regarding the usefulness of this technology.
The paucity of evidence and clear guidelines regarding transfusion thresholds in TBI patients was identified as a critical factor limiting the adoption of transfusion strategies by clinicians. This highlights the need for appropriate clinical trials to inform optimal transfusion practices in TBI.21,26 Our survey emphasizes the clinical equipoise regarding transfusion strategies of physicians caring for TBI patients and the lack of information regarding their relationship to clinical outcome.
Our study has several limitations. First, the response rate was lower than expected, although it is comparable with other published international critical care surveys.34 To be exhaustive in targeting our population of interest, we considered every critical care physician and neurosurgeon working in trauma centres. We may have included potential respondents that do not care for TBI patients, leading to an underestimation of our response rate. In the past, self-administered and web-based surveys have typically generated lower response rates than other types of surveys.35,36 To help mitigate this, repeated reminders were sent to those who did not respond. We felt that the majority of clinicians would be more comfortable with the use of web-based media than they were a few years ago. Second, the data from our survey represent self-reported beliefs of practice and do not necessarily reflect actual practices. Nevertheless, surveys based on clinical scenarios do reflect actual clinical practice, thus potentially reducing this type of response bias.37,38 Third, our survey explores a very specific topic that may not have resonated with all who answered. Nevertheless, most of our respondents were experienced physicians working in high-volume TBI centres. Fourth, our study, like all surveys, did not allow us to measure all determinants that could theoretically influence a physician’s practice. For example, patients and relatives can influence decisions regarding RBC transfusions because of their religious or cultural beliefs, and by previously agreed to level of care decisions. In addition, respondents may have been influenced by regional or institutional norms and protocols, generating a potential cluster effect.
In summary, our international survey of critical care physicians and neurosurgeons indicates that RBC transfusion of critically ill TBI patients is influenced by patient characteristics and neuro-monitoring. We did not identify a specific consensus threshold for which respondents believed an RBC transfusion should be administered. Clinical equipoise regarding an optimal transfusion strategy is attributed to lack of clear clinical evidence and guidelines. Robust multicenter clinical trials evaluating transfusion strategies in critically ill patients with TBI are necessary to establish an adequate level of evidence and best-practice guidelines.
References
Boutin A, Moore L, Lauzier F, et al. Transfusion of red blood cells in patients with traumatic brain injuries admitted to Canadian trauma health centres: a multicentre cohort study. BMJ Open 2017; 7: e014472.
Corwin HL, Gettinger A, Pearl RG, et al. The CRIT study: anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit Care Med 2004; 32: 39-52.
Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA 2002; 288: 1499-507.
Miller JD, Sweet RC, Narayan R, Becker DP. Early insults to the injured brain. JAMA 1978; 240: 439-42.
Salim A, Hadjizacharia P, DuBose J, et al. Role of anemia in traumatic brain injury. J Am Coll Surg 2008; 207: 398-406.
Boutin A, Moore L, Green RS, et al. Hemoglobin thresholds and red blood cell transfusion in adult patients with moderate or severe traumatic brain injuries: a retrospective cohort study. J Crit Care 2018; 45: 133-9.
Van Beek JG, Mushkudiani NA, Steyerberg EW, et al. Prognostic value of admission laboratory parameters in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007; 24: 315-28.
Yamal JM, Rubin ML, Benoit JS, et al. Effect of hemoglobin transfusion threshold on cerebral hemodynamics and oxygenation. J Neurotrauma 2015; 32: 1239-45.
Leal-Noval SR, Munoz-Serrano A, Arellano-Orden V, et al. Effects of red blood cell transfusion on long-term disability of patients with traumatic brain injury. Neurocrit Care 2016; 24: 371-80.
Smith MJ, Stiefel MF, Magge S, et al. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med 2005; 33: 1104-8.
Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med 2009; 37: 1074-8.
Duane TM, Mayglothling J, Grandhi R, et al. The effect of anemia and blood transfusions on mortality in closed head injury patients. J Surg Res 2008; 147: 163-7.
Warner MA, O’Keeffe T, Bhavsar P, et al. Transfusions and long-term functional outcomes in traumatic brain injury. J Neurosurg 2010; 113: 539-46.
Elterman J, Brasel K, Brown S, et al. Transfusion of red blood cells in patients with a prehospital Glasgow Coma Scale score of 8 or less and no evidence of shock is associated with worse outcomes. J Trauma Acute Care Surg 2013; 75: 8-14.
Lacroix J, Hébert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007; 356: 1609-19.
Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340: 409-17.
Carson JL, Carless PA, Hebert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA 2013; 309: 83-4.
Carson JL, Stanworth SJ, Roubinian N, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2016; 10: CD002042.
Carlson AP, Schermer CR, Lu SW. Retrospective evaluation of anemia and transfusion in traumatic brain injury. J Trauma 2006; 61: 567-71.
George ME, Skarda DE, Watts CR, Pham HD, Beilman GJ. Aggressive red blood cell transfusion: no association with improved outcomes for victims of isolated traumatic brain injury. Neurocrit Care 2008; 8: 337-43.
Desjardins P, Turgeon AF, Tremblay MH, et al. Hemoglobin levels and transfusions in neurocritically ill patients: a systematic review of comparative studies. Crit Care 2012; 16: R54.
Robertson CS, Hannay HJ, Yamal JM, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA 2014; 312: 36-47.
Sena MJ, Rivers RM, Muizelaar JP, Battistella FD, Utter GH. Transfusion practices for acute traumatic brain injury: a survey of physicians at US trauma centers. Intensive Care Med 2009; 35: 480-8.
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury. Fourth Edition. Neurosurgery 2017; 80: 6-15.
Turgeon AF, Lauzier F, Simard JF, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ 2011; 183: 1581-8.
Boutin A, Chassé M, Shemilt M, et al. Red blood cell transfusion in patients with traumatic brain injury: a systematic review and meta-analysis. Transfus Med Rev 2016; 30: 15-24.
Burns KE, Duffett M, Kho ME, et al. A guide for the design and conduct of self-administered surveys of clinicians. CMAJ 2008; 179: 245-52.
Bryson GL, Turgeon AF, Choi PT. The science of opinion: survey methods in research. Can J Anesth 2012; 59: 736-42.
Powell C. The Delphi technique: myths and realities. J Adv Nurs 2003; 41: 376-82.
The American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 7th ed. Deerfield, IL: AAPOR; 2011 .
Yang Z, Zhou M. Kappa statistic for clustered matched-pair data. Stat Med 2014; 33: 2612-33.
Badenes R, Oddo M, Suarez JI, et al. Hemoglobin concentrations and RBC transfusion thresholds in patients with acute brain injury: an international survey. Crit Care 2017; 21: 159.
Kramer AH, Diringer MN, Suarez JI, Naidech AM, Macdonald LR, Le Roux PD. Red blood cell transfusion in patients with subarachnoid hemorrhage: a multidisciplinary North American survey. Crit Care 2011; 15: R30.
Karam O, Tucci M, Lacroix J, Rimensberger PC; Canadian Critical Care Trials Group and of the Pediatric Acute Lung Injury Sepsis Investigator Network. International survey on plasma transfusion practices in critically ill children. Transfusion 2014; 54: 1125-32.
Manfreda KL, Berzelak J, Vehovar V, Bosjnak M, Haas I. Web surveys versus other survey modes: a meta-analysis comparing response rates. Int J Market Res 2008; 50: 79-104.
Groves RM, Fowler FJ Jr, Couper MP, Lepkowski JM, Singer E, Tourangeau R. Survey Methodology. New York, NY: Wiley; 2004 .
Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA 2000; 283: 1715-22.
Peabody JW, Luck J, Glassman P, et al. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med 2004; 141: 771-80.
Acknowledgements
The authors would like to thank Xavier Neveu MSc for his help with statistical analyses, and Caroline Léger PhD and Marjorie Daigle for their administrative help. This study was developed with the Canadian Critical Care Trials Group (CCCTG) and the Canadian Traumatic Brain Injury Research Consortium (CTRC). We want to thank the CCCTG and the CTRC Grants and Manuscripts Committees for critically reviewing the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Editorial responsibility
This submission was handled by Dr. Steven Backman, Associate Editor, Canadian Journal of Anesthesia.
Author contributions
Paule Lessard Bonaventure and Alexis F. Turgeon contributed to the study concept and design. Paule Lessard Bonaventure, Michèle Shemilt, Amélie Robitaille, and Alexis F. Turgeon contributed to the acquisition, analysis, and interpretation of data. Paule Lessard Bonaventure and Alexis F. Turgeon contributed to drafting the manuscript. Paule Lessard Bonaventure, Michèle Shemilt, and Alexis F. Turgeon contributed to the statistical analyses. All authors contributed to the critical revision of the manuscript. Alexis F. Turgeon contributed to the study supervision.
Funding
This work was funded by the CHU de Québec – Université Laval Foundation (Killimanjaro Fund) and by a Foundation Scheme Grant (354039) from the Canadian Institutes of Health Research (CIHR). Dr Lessard Bonaventure is the recipient of a training award from the CIHR. Drs. Lauzier, Moore, and Chassé are recipients of a research career award from the Fonds de Recherche du Québec - Santé (FRQS). Drs. Turgeon, Lauzier, and Moore are supported by the Traumatology Research Consortium of the FRQS. Dr. Turgeon is the chairholder of the Canada Research Chair in Critical Neurology and Trauma. The Canadian Traumatic Brain Injury Research Consortium is funded by a Team Grant from the CIHR, and the Canadian Critical Care Trials Group is funded by a Community Development Grant from the CIHR.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix

Rights and permissions
About this article
Cite this article
Lessard Bonaventure, P., Lauzier, F., Zarychanski, R. et al. Red blood cell transfusion in critically ill patients with traumatic brain injury: an international survey of physicians’ attitudes. Can J Anesth/J Can Anesth 66, 1038–1048 (2019). https://doi.org/10.1007/s12630-019-01369-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01369-w