Abstract
Background
Nitrous oxide (N2O) has been reported to increase the risk of postoperative nausea and vomiting (PONV) in a dose-dependent manner. We investigated the effect of adding N2O at the end of isoflurane inhalational anesthesia on the recovery and incidence of PONV. Our hypothesis was that N2O would reduce the time to early recovery without increasing the incidence of PONV.
Methods
After obtaining ethics committee approval and written informed consent, 100 women at American Society of Anesthesiologists physical status I-III and scheduled for laparoscopic-assisted vaginal hysterectomy were randomized into two groups (G) according to the carrier gas: GO2 (air in 30% oxygen) and GN2O (the same mixture until the last 30 min of surgery, when 70% N2O in 30% oxygen was used). No PONV prophylaxis was given. Anesthesia was induced with thiopental 5 mg·kg−1, vecuronium 0.1 mg·kg−1, and fentanyl 1-2 μg·kg−1 iv and maintained with isoflurane. Indicators of early recovery (time to extubation, eye opening, following commands, orientation) were assessed by an anesthesiologist unaware of the group assignment. The incidence and severity of PONV was measured at two and 24 hr postoperatively.
Results
Altogether, 82 participants completed the study (42 in GO2, 40 in GN2O) and were analyzed. The mean (SD) time of N2O administration in GN2O patients was 27.1 (10.1) min. The mean (SD) time to extubation was faster in GN2O patients [5.4 (2.9) min] than in GO2 patients [7.5 (3.7) min] (mean difference, 2.0 min; 95% confidence interval [CI], 0.6 to 3.4, P = 0.009). The ability to open eyes, follow commands, and being oriented were all faster in GN2O patients than in GO2 patients (differences of 3.9 min, 95% CI, 1.6 to 6.1, P = 0.001; 3.4 min, 95% CI, 1.0 to 5.7, P = 0.006; 3.8 min, 95% CI, 0.9 to 6.7, P = 0.010, respectively). The incidence of PONV was not different between the groups, but the rescue antiemetic was required less often in the GN2O patients (mean difference in metoclopramide dose between the GN2O and GO2 groups, 5.1 mg; 95% CI, 0.8 to 9.4, P = 0.019).
Conclusions
Adding N2O during the last 30 min of an isoflurane-based inhalational anesthetic reduced the time to extubation, eye opening, and orientation.
Résumé
Contexte
Selon des études, le protoxyde d’azote (N2O) augmenterait le risque de nausées et vomissements postopératoires (NVPO) de façon dose-dépendante. Nous avons évalué l’effet d’un ajout de N2O à la fin d’une anesthésie par inhalation d’isoflurane sur la récupération et l’incidence de NVPO. Notre hypothèse était que le N2O réduirait le temps de récupération précoce sans augmenter l’incidence de NVPO.
Méthode
Après avoir obtenu l’approbation du comité d’éthique et le consentement éclairé écrit, 100 femmes de statut physique I-III selon l’American Society of Anesthesiologists devant subir une hystérectomie vaginale assistée par laparoscopie ont été réparties en deux groupes (G) selon le gaz de transport : GO2 (air avec 30 % oxygène) et GN2O (le même mélange jusqu’aux 30 dernières min de chirurgie, puis 70 % N2O avec 30% oxygène). Aucune prophylaxie anti-NVPO n’a été administrée. L’anesthésie a été induite à l’aide de 5 mg·kg−1 de thiopental, 0,1 mg·kg−1 de vécuronium, et 1-2 µg·kg−1 de fentanyl iv, et maintenue à l’aide d’isoflurane. Les indicateurs de récupération précoce (soit le temps jusqu’à l’extubation, jusqu’à l’ouverture des yeux, à la réponse aux ordres, à l’orientation) ont été évalués par un anesthésiologiste ignorant l’attribution de groupe. L’incidence et la gravité des NVPO ont été mesurées à deux et 24 h postopératoires.
Résultats
Au total, 82 participantes ont terminé l’étude (42 dans le groupe GO2, 40 dans le groupe GN2O) et leurs résultats ont été analysés. La durée moyenne (ÉT) d’administration de N2O chez les patientes du groupe GN2O était de 27,1 (10,1) min. Le temps moyen (ÉT) jusqu’à l’extubation était plus court chez les patientes du groupe GN2O [5,4 (2,9) min] que chez celles du groupe GO2 [7,5 (3,7) min] (différence moyenne, 2,0 min; intervalle de confiance [IC] 95 %, 0,6 à 3,4, P = 0,009). La capacité à ouvrir les yeux, à obéir à des ordres et à s’orienter se sont rétablies plus rapidement dans le groupe GN2O que dans le groupe GO2 (différences de 3,9 min, IC 95 %, 1,6 à 6,1, P = 0,001; 3,4 min, IC 95 %, 1,0 à 5,7, P = 0,006; 3,8 min, IC 95 %, 0,9 à 6,7, P = 0,010, respectivement). L’incidence de NVPO n’était pas différente entre les groupes, mais l’antiémétique de sauvetage a été moins souvent requis dans le groupe GN2O (différence moyenne de dose de métoclopramide entre les groupes GN2O et GO2, 5,1 mg; IC 95 %, 0,8 à 9,4, P = 0,019).
Conclusion
L’ajout de N2O au cours des 30 dernières minutes d’une anesthésie par inhalation d’isoflurane a réduit le délai jusqu’à l’extubation, l’ouverture des yeux et l’orientation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The older volatile anesthetic (VA) isoflurane is less expensive per milliliter than the newer VAs, such as sevoflurane and desflurane. Isoflurane’s higher blood/gas solubility, however, increases the context decrement time, resulting in longer recovery from anesthesia. A meta-analysis showed that patients followed commands, on average, 4.4 min earlier with desflurane than with isoflurane.1 In longer cases, when the total dose of VA is significantly higher and wake-up time is more prolonged, it may seem logical to use an older, less expensive VA with higher blood/gas solubility for anesthesia maintenance and then switch to a more costly VA with a faster wake-up profile towards the end of anesthesia. A study of volunteers undergoing general anesthesia, however, showed that switching from isoflurane to desflurane at the end of the anesthesia did not hasten recovery.2
Another option to reduce the overall consumption of VA is to add nitrous oxide (N2O) as a carrier gas. Nitrous oxide has been used for its minimum alveolar concentration (MAC) sparing effect and accelerates awakening after general anesthesia.3 Nevertheless, N2O has side effects, such as a risk of postoperative nausea and vomiting (PONV) in a dose-dependent manner and with prolonged duration.4,5 Some anesthesia providers choose to add N2O at the end of anesthesia to hasten awakening and extubation in the operating room (OR), but this practice has not been well investigated.
We therefore investigated the addition of N2O towards the end of isoflurane anesthetic regarding its influence on the time to awakening and the appearance of PONV. We hypothesized that the addition of N2O 30 min before the expected end of surgery would reduce the time to early recovery without increasing the incidence of PONV.
Methods
After obtaining approval of the Ethical Committee of General Hospital Zadar, Croatia (IRB approval 01-2661-2/06) and written informed consent from the patients, we enrolled 100 adult women at American Society of Anesthesiologists (ASA) physical status I-III in the study who were to undergo general anesthesia for elective laparoscopic-assisted vaginal hysterectomy.
We excluded subjects with potentially confounding factors that could increase the risk of PONV and/or prolong routine laparoscopic surgery. Exclusion criteria were pregnancy; recently having given birth or within less than two months after termination of pregnancy; breastfeeding; obesity (body mass index > 30 kg·m−2); known hypersensitivity to drugs used in the study protocol; use of antiemetics, psychotropic drugs, hormones, and/or steroids within 72 hr before surgery; nausea and/or vomiting immediately prior to surgery; history of multiple laparotomies; diseases that impair gastric motility (diabetes mellitus, chronic cholecystitis, gastric and intestinal disease, neuromuscular disorders, neuropathies, liver dysfunction); vestibular disease; history of migraine headaches, central nervous system injury, renal impairment, alcoholism, and/or opioid addiction. Subjects were also excluded (post-randomization) if they developed conditions that could influence the incidence of PONV, postoperative pain or morbidity (e.g., significant intraoperative surgery complications), intraoperative drug allergy, severe intraoperative hypotension, perioperative hypoxia, excessive blood loss, difficult intubation, or if the surgery had been converted to an open approach.
Preoperatively, all subjects fasted at least six hours but were allowed to drink clear fluids up to two hours before the surgery and received midazolam 7.5 mg po one hour before the surgery. No prophylactic antiemetics were given as it was the hospital’s standard procedure at the time of the study. Standard intraoperative monitoring included electrocardiography, noninvasive blood pressure, pulse oximetry, and capnography. Anesthesia was induced with thiopental 5 mg·kg−1, fentanyl 1-2 μg·kg−1, and vecuronium 0.1 mg·kg−1 iv. All subjects received 500 mL of saline prior to surgery and crystalloids 10 mL·kg−1·hr−1 during surgery. The lungs were manually ventilated via face mask with oxygen 6 L·min−1 for three minutes before the trachea was intubated.
Subjects were randomized into the two groups by computer-generated random numbers. Each allocation number was concealed in an opaque envelope before the start of the surgery and was revealed 30 min before the end of the operation. The oxygen group (GO2) received air in 30% O2 throughout the procedure. The nitrous oxide group (GN2O) received the same carrier gas mixture until the last 30 min of surgery, when 70% N2O in 30% O2 was administered. Anesthesia was maintained with isoflurane at ~1 MAC delivered in fresh gas flow at 3 L·min−1.
In GO2 patients, the end-tidal concentration of isoflurane was maintained at ~1 MAC. Isoflurane was discontinued at the start of skin closure. In GN2O patients, N2O was started 30 min before skin closure, with the concentration adjusted to keep the total anesthetic concentration (isoflurane + N2O) at ~1 MAC. Both isoflurane and N2O were discontinued at the start of skin closure. Supplemental bolus doses of fentanyl (1 μg·kg−1 iv) were given to keep the heart rate and blood pressure within 20% of baseline values in both groups, and additional doses of vecuronium were administered to maintain one to two twitches on the train-of-four neuromuscular monitor.
The lungs were mechanically ventilated to maintain normocapnia (end-tidal CO2 36-38 mmHg). A nasogastric tube was not placed. All laparoscopy-assisted operations were performed with CO2 insufflation to an intraabdominal pressure of 15 mmHg. At the end of the surgery, the fresh gas inflow rate was changed to 6 L·min−1 of 100% O2, and the neuromuscular blockade was reversed (neostigmine 2.5 mg and atropine 1 mg iv) in all subjects.
Extubation criteria were standardized to meet the usual criteria for extubation in our hospital. Subjects were extubated when the spontaneous tidal volume was > 300 mL, respiratory rate was > 8 breaths·min−1, and end-tidal CO2 was ≤ 45 mmHg. The patients were monitored in the OR until they opened their eyes and followed commands. Early recovery (time to extubation, eye opening, following verbal commands, orientation to time and place) was recorded by an anesthesiologist blinded to the anesthesia technique. Simple verbal orders (“open your eyes”, “squeeze my hand”, “open your mouth”) were repeated every 15 sec.
Postoperatively, subjects received crystalloids 5 mL·kg−1·hr−1 and were allowed to drink water after three hours, if tolerated. All subjects stayed in the hospital for at least 24 hr.
The incidence of postoperative nausea (PON), vomiting (POV), or PONV and the use of rescue antiemetics were recorded at two and 24 hr after surgery. The severity of PON and pain were evaluated using a 100-mm visual analogue scale (VAS; 0 = no pain/nausea, 100 = maximum pain/nausea) at the same time points. The postoperative nausea VAS score was recorded for each episode, with the highest score used for statistical analysis. Subjects were considered to have PONV if they experienced at least one episode of nausea (VAS > 0), vomiting, or retching, or any combination of these events. Postoperative vomiting was defined as at least one episode of vomiting or retching during the initial 24 hr postoperatively. Postoperative nausea and vomiting, PON, and POV were defined as early (during the first two hours, in the postoperative recovery area) or late (during the first 2-24 postoperative hours, on the ward). The same anesthesiologist blinded to the anesthesia technique collected all postoperative data. A rescue antiemetic (metoclopramide 0.4 mg·kg−1 iv) was given to those who experienced two or more episodes of vomiting and/or retching within a period of 30 min, any nausea lasting > 15 min, nausea VAS score ≥ 50 mm, or when requested for PONV management. The pain VAS score and total dose of postoperative opioids were also recorded at two and 24 hr after surgery. Diclofenac 75 mg im was given immediately after surgery and, if needed, 12 hr later. For severe pain (VAS > 40 mm) meperidine 50 to 100 mg iv was used and repeated every four hours, as needed.
The sample size for recovery time (primary endpoint) was calculated on the assumption that 15 (SD 7) min would be needed for early recovery (following commands) for the GO2 patients and that the GN2O patients would require four minutes less time (11 [SD 5] min). We would need 38 patients in each group for the primary outcome to have a power of 0.8 and an alpha level of 0.05. The study was also designed to have adequate power for the secondary endpoint, the incidence of PONV. The sample size for PONV was calculated based on the results from our previous study, which had a 30% incidence of PONV among the GO2 patients and 60% among the GN2O patients.4 At least 40 subjects per group would be needed to show the difference between groups with a power of 0.8 and an alpha level of 0.05. Although the incidences we used for the power calculation of PONV in this study were much higher than those reported in the literature – e.g., ENIGMA II (11% vs 15 %) and IMPACT (31% vs 35%) trials6,7 – the incidence was based on a previously reported study conducted on the same surgical population under similar conditions.4 The Chi-square and Mann-Whitney tests were used to analyze the data. P < 0.05 was considered to indicate statistical significance.
Results
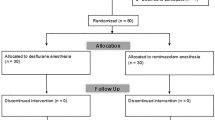
Overall, 82 of the 100 subjects enrolled between September 2006 and November 2010 completed the study. The Figure presents a flow chart of the study enrolment and reasons for exclusion from the analysis. There were no differences between the two groups regarding the participants’ age, body mass index, ASA physical status, smoking status, history of PONV and/or motion sickness, probability of PONV using the Apfel risk score, duration of anesthesia and surgery, use of the induction agent, or intraoperative use of fentanyl (Table 1). On average, the GN2O patients received N2O at the end of anesthesia for 27 (SD 10) min. The GN2O patients recovered significantly faster than the GO2 patients. They were, on average, extubated two minutes earlier, and they opened their eyes, followed commands, and became oriented about four minutes more rapidly (Table 2).
All subjects in both groups received diclofenac at the end of the surgery. Although there was no difference in the amount of meperidine and additional diclofenac administered to the two groups, the GN2O patients had less pain at two hours postoperatively (Table 2). Furthermore, one in six of the GN2O patients did not require postoperative opioids, whereas all of the GO2 patients were given at least one dose of meperidine (Table 2). At 24 hr, there was no difference in pain between the groups (Table 2). Faster early recovery was not accompanied by an increased risk of PONV. There were no differences between groups at any point regarding nausea and/or vomiting (Table 3). Moreover, the GN2O patients received significantly less metoclopramide (Table 3). No adverse events were noted during the study.
Discussion
This study suggested that adding 70% N2O during the last 30 min of general anesthesia maintained with isoflurane hastened extubation and early recovery without increasing the incidence of PONV in patients undergoing laparoscopic gynecologic surgery. This practice may reduce pain scores at two hours postoperatively and the overall need of postoperative opioids.
Thus, in our opinion, the clinical importance of the results of this study for anesthesia providers who routinely use isoflurane is that they could add N2O at the end of surgery without worrying about increasing the risk of PONV and achieve recovery times similar to those when sevoflurane or desflurane is used. For anesthesia providers who do not use isoflurane, our results indicate that they could consider using the isoflurane/N 2O at the end (ISONATE) technique without concerns of increased morbidity/PONV and with recovery times similar to those achieved using sevoflurane alone.
Although isoflurane is no longer frequently used in North America, it remains the predominant VA used worldwide. A recent study of cardiac surgery showed that sevoflurane was not superior to isoflurane regarding any important clinical outcomes.8 The study also calculated cost savings if isoflurane was used for a 4.5-hr operation ($5.25 vs $41.24, isoflurane vs sevoflurane, respectively), potentially saving US$10 million in the Unites States alone if isoflurane was used instead of sevoflurane for all cardiac operations.8 The savings would clearly be greater if isoflurane was used instead of sevoflurane for general surgery. These figures suggest that isoflurane might not yet be obsolete and that clinicians should consider using the ISONATE technique.
The faster early recovery in the N2O group in our study (orientation to time and place was 3.8 min faster) was comparable to the results of a meta-analysis comparing desflurane with isoflurane anesthesia (4.4 min).1 In another recent meta-analysis, the percentage reductions in time to extubation were 34% (95% confidence interval [CI] 28 to 39) for desflurane and 13% (95% CI 1 to 23) for sevoflurane,9 which are comparable to our results of 27% (95% CI 18 to 38). These percentages suggest that adding N2O at the end of isoflurane anesthesia may result in a clinical response similar to the early recovery seen when using sevoflurane or desflurane.
Nitrous oxide has been used for years with a relatively good safety record, although its safety was recently challenged by two large multicenter trials.5,9 In the ENIGMA trial, with 2,050 patients, the N2O-free patients had a significantly lower combined rate of major complications and lower incidence of severe PONV.5 In the ENIGMA-II trial, with > 7,000 patients, N2O avoidance did not reduce the incidence of major perioperative cardiac events and death.7,10
Nitrous oxide emetic properties had been controversial until the large, multifactorial IMPACT trial confirmed that use of N2O increased the risk of PONV.6 This difference in results could be partly explained not only by the different inspiratory concentrations of N2O used in studies but also the different durations of N2O administration. Hence, the total amount of N2O delivered (concentration + duration) to the patient may be important.
Recently, it was suggested that the risk of PONV is increased with an increased inspiratory concentration of N2O.4 When the N2O inspiratory concentration was increased from 50% to 70%, PONV increased from 46% to 62%. There is a paucity of data on the duration of N2O exposure and PONV. A recent meta-analysis combined the data of 29 studies (> 10,000 patients) and found that the risk of PONV increased by 20% per hour of N2O exposure after the first 45 min.11 The statistical analysis, however, was later questioned.12 Moreover, the inspiratory N2O concentrations were not taken into account for either the analysis or the stratification by the type of surgery (the majority of shorter-duration surgeries were gynecological).13 Only one published study specifically explored the duration of anesthesia with N2O and PONV.14 The study was small (30 patients in the N2O group, 154 in the O2 group), and randomization could have been biased (only one anesthesia provider was using N2O; the other two providers did not).14 In addition, the N2O inspiratory concentration was not standardized. The results of the study suggested that exposure to N2O for three hours or more could increase the risk for PONV compared with a shorter exposure.14 In our study, we chose to add N2O for 30 min because a shorter duration of N2O administration might not have a detrimental effect on PONV. Indeed, we used N2O for only 27 ± 10 min on average and found no difference in PONV between the two groups. It appears that the short exposure of 70% N2O is not long enough to trigger the emetic mechanism.
The mechanism of N2O-associated PONV has not been well studied, but it could include an increase in the middle ear pressure, bowel distension, activation of the dopaminergic system in the chemoreceptor trigger zone, and interaction with opioid receptors. In our study, not only was there no difference in PONV between groups, the participants in the oxygen group required more rescue antiemetic. Also, more subjects were treated for PONV (62%, 26/42) than those who received N2O (40%, 16/40). One of the explanations for this surprising result could be that significantly fewer subjects in the N2O group required postoperative opioids (17.5% vs 0%: GO2 vs GN2O, P = 0.005). Additionally, those who received N2O at the end of anesthesia required less postoperative opioids and had significantly less pain at two hours postoperatively. Our data support the ENIGMA trial results, which suggested that N2O might have a postoperative analgesic effect.15 The retrospective review of ENIGMA subjects showed a shorter use of patient-controlled analgesia in the N2O group but no difference in pain scores or opioid usage.
The mechanism of N2O analgesic action is not clearly understood. N2O acts on various receptors at different sites of the central nervous system (dopaminergic, glutamatergic, adrenergic, benzodiazepine), but its activity as an N-methyl-D-aspartate antagonist may be important in preventing central sensitization.16 Shorter exposure to N2O could have more effect on postoperative pain than a longer exposure because more N2O exposure could induce acute tolerance and diminish its postoperative analgesic and opioid sparing effect. Indeed, a study in healthy volunteers exposed to 60-80% of N2O for three hours found that the maximum analgesic effect was observed after 20-30 min of exposure to N2O. It then gradually decreased and was completely absent in all volunteers within 150 min.17
There are several limitations in our study. First, this study was conducted in a single centre with only one type of surgical procedure, the duration of which was about two hours. Therefore, the results may not be generalized to other types of surgery or for a longer duration of isoflurane anesthesia because the isoflurane context decrement time increases more steeply after 100 min of delivery. This could significantly prolong recovery compared with a shorter exposure. Adding N2O at the end of several hours of isoflurane anesthesia could potentially hasten recovery even more because of the second gas effect on emergence from anesthesia.18 A larger, multicenter study with a longer duration of anesthesia would be needed to confirm this point. Using the PONV incidences in the ENIGMA II and IMPACT trials, 2,815 and 1,271 subjects per group, respectively, would be needed to show that adding N2O at the end of anesthesia would increase the PONV incidence with a power of 0.8 and P < 0.05.6,7
Second, PONV prophylaxis was not given to subjects in either group as it was the standard of care in our hospital at the time of the study. Although administering the same antiemetic prophylaxis in both groups would likely decrease the incidence of PONV in both groups, it would not have an influence on extubation or orientation times. When PONV prophylaxis was used in the ENIGMA II trial, there was no significant trend to increase the incidence of severe PONV using N2O (13.1% vs 11.8%).7 Assuming the same incidence, a study of 11,539 subjects per group would be needed to show the difference with a power of 0.8.
Third, the difference in extubation time between our study groups was modest (two minutes). It was done at a “deeper” extubation stage, however, when subjects met ventilation criteria for the extubation (the standard procedure in our hospital at the time of the study). We did not want to change the hospital protocol for this study. If extubation had been performed after the subjects were following commands, it could be expected that the difference would be at least as much as the difference in the time for following commands (3.5 min), if not longer.
In summary, adding 70% N2O during the last 30 min of isoflurane-maintained anesthesia hastens early recovery without increasing the incidence of PONV. It may also be associated with lower pain scores and less opioid use during the early recovery period.
References
Dexter F, Tinker JH. Comparisons between desflurane and isoflurane or propofol on time to following commands and time to discharge. A metaanalysis. Anesthesiology 1995; 83: 77-82.
Neumann MA, Weiskopf RB, Gong DH, Eger EI 2nd, Ionescu P. Changing from isoflurane to desflurane toward the end of anesthesia does not accelerate recovery in humans. Anesthesiology 1998; 88: 914-21.
Peyton PJ, Chao I, Weinberg L, Robinson GJ, Thompson BR. Nitrous oxide diffusion and the second gas effect on emergence from anesthesia. Anesthesiology 2011; 114: 596-602.
Mraovic B, Simurina T, Sonicki Z, Skitarelic N, Gan TJ. The dose-response of nitrous oxide in postoperative nausea in patients undergoing gynecologic laparoscopic surgery: a preliminary study. Anesth Analg 2008; 107: 818-23.
Myles PS, Leslie K, Chan MT, et al; ENIGMA Trial Group. Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology 2007; 107: 221-31.
Apfel CC, Korttila K, Abdalla M, et al; IMPACT Investigators. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 2004; 350: 2441-51.
Myles PS, Chan MT, Kasza J, et al. Severe nausea and vomiting in the evaluation of Nitrous Oxide in the Gas Mixture for Anesthesia II Trial. Anesthesiology 2016; 124: 1032-40.
Jones PM, Bainbridge D, Chu MW, et al. Comparison of isoflurane and sevoflurane in cardiac surgery: a randomized non-inferiority comparative effectiveness trial. Can J Anesth 2016; 63: 1128-39.
Agoliati A, Dexter F, Lok J, et al. Meta-analysis of average and variability of time to extubation comparing isoflurane with desflurane or isoflurane with sevoflurane. Anesth Analg 2010; 110: 1433-9.
Myles PS, Leslie K, Peyton P, et al; ANZCA Trials Group. Nitrous oxide and perioperative cardiac morbidity (ENIGMA-II) trial: rationale and design. Am Heart J 2009; 157: 488-94.
Peyton PJ, Wu CY. Nitrous oxide-related postoperative nausea and vomiting depends on duration of exposure. Anesthesiology 2014; 120: 1137-45.
Pace NL. Questioning a relationship between nitrous oxide duration of exposure and postoperative nausea and vomiting. Anesthesiology 2014; 121: 1356-8.
Zhou L, Chen C, Yu H. Nitrous oxide-related postoperative nausea and vomiting depends on duration of exposure: more questions than answers. Anesthesiology 2014; 121: 1356.
Smiley BA, Paradise NF. Does the duration of N2O administration affect postoperative nausea and vomiting? Nurse Anesth 1991; 2: 13-8.
Stiglitz DK, Amaratunge LN, Konstantatos AH, Lindholm DE. Intraoperative nitrous oxide as a preventive analgesic. Anaesth Intensive Care 2010; 38: 890-3.
Richebe P, Rivat C, Creton C, et al. Nitrous oxide revisited: evidence for potent antihyperalgesic properties. Anesthesiology 2005; 103: 845-54.
Rupreht J, Dworacek B, Bonke B, Dzoljic MR, van Eijndhoven JH, de Vlieger M. Tolerance to nitrous oxide in volunteers. Acta Anaesthesiol Scand 1985; 29: 635-8.
Eger EI 2nd, Shafer SL. Tutorial: context-sensitive decrement times for inhaled anesthetics. Anesth Analg 2005; 101: 688-96.
Acknowledgements
The authors appreciate greatly the help of Dr. Simon Mikulandra from Ordensklinikum Linz – Elisabethinen, Linz, Austria with the statistical analysis.
Conflicts of interest
Tong J. Gan: current and previous research grant support and honoraria from Fresenius Kabi, Mallinckrodt, Merck, Pacira, and Edwards. None of the above companies manufacture or market nitrous oxide.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Associate Editor, Canadian Journal of Anesthesia.
Author contributions
Boris Mraovic helped with the conception and design of the study, data analysis, interpretation of the data. He also drafted and prepared the manuscript and approved the final manuscript. Boris Mraovic attests to the integrity of the original data and the analysis reported in this manuscript. Tatjana Simurina helped with conception and design of the study; conducted the study; collected and analysed data; prepared the manuscript; and approved the final manuscript. Tatjana Simurina attests to the integrity of the original data and the analysis reported in the manuscript. Tatjana Simurina is the archival author. Tong J. Gan helped prepare the manuscript and approved the final manuscript.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is accompanied by an editorial. Please see Can J Anesth 2018; 65: this issue.
Rights and permissions
About this article
Cite this article
Mraovic, B., Simurina, T. & Gan, T.J. Nitrous oxide added at the end of isoflurane anesthesia hastens early recovery without increasing the risk for postoperative nausea and vomiting: a randomized clinical trial. Can J Anesth/J Can Anesth 65, 162–169 (2018). https://doi.org/10.1007/s12630-017-1013-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-1013-y