Abstract
Purpose
Maintenance of a remifentanil infusion during anesthetic emergence has been reported to decrease the incidence of coughing and thereby help to ensure a smooth emergence. It may, however, cause respiratory depression and possibly delay emergence. The purpose of this study was to investigate the effect of a single dose of dexmedetomidine combined with a low-dose remifentanil infusion on cough suppression during emergence from general anesthesia.
Methods
American Society of Anesthesiologists physical status I-II adults undergoing elective thyroidectomy under sevoflurane anesthesia were recruited and randomly allocated to receive either dexmedetomidine 0.5 μg·kg−1 iv (Group D, n = 70) or saline (Group S, n = 71), each combined with a low-dose remifentanil infusion ten minutes before the end of surgery. Coughing was assessed using a four-point scale. The respiratory rate (RR), heart rate (HR), and mean arterial pressure were also recorded.
Results
The incidence of coughing was lower in Group D than in Group S (64% vs 91%, respectively; mean difference 27%; 95% confidence interval [CI] 13 to 41; P < 0.001). The median cough grade at extubation was also lower in Group D. Mean arterial pressure and HR were elevated in Group S during tracheal extubation but were similar to baseline values in Group D. There was no difference in RR between the two groups throughout the study. A small delay in extubation was observed in Group D (3 minutes longer than Group S; 95% CI 2 to 4; P < 0.001).
Conclusion
Compared with an infusion of low-dose remifentanil alone, the addition of a single dose (0.5 μg·kg−1) of dexmedetomidine during emergence from sevoflurane-remifentanil anesthesia was effective in attenuating coughing and hemodynamic changes and did not exacerbate respiratory depression after thyroid surgery. This trial was registered at Clinicaltrial.gov, identifier: NCT01774305.
Résumé
Objectif
Il a été rapporté que le maintien d’une perfusion de remifentanil pendant l’émergence anesthésique diminuait l’incidence de la toux et, par conséquent, contribuait à assurer une émergence en douceur. Elle peut toutefois provoquer une dépression respiratoire et possiblement retarder l’émergence. L’objet de cette étude était d’analyser l’effet d’une dose unique de dexmédétomidine combinée à une perfusion de remifentanil à faible dose sur la suppression de la toux au cours de l’émergence d’une anesthésie générale.
Méthodes
Des patients adultes répondant au statut physique I-II de l’American Society of Anesthesiologists et subissant une thyroïdectomie élective sous anesthésie au sévoflurane ont été recrutés et randomisés pour recevoir de la dexmédétomidine 0,5 μg·kg−1 IV (Groupe D, n = 70) ou du sérum physiologique (Groupe S, n = 71) en association avec une perfusion de remifentanyl à faible dose, dix minutes avant la fin de la chirurgie. La toux a été évaluée au moyen d’une échelle à quatre points. La fréquence respiratoire (FR), la fréquence cardiaque (FC) et la tension artérielle moyenne ont été également enregistrées.
Résultats
L’incidence de la toux a été plus faible dans le Groupe D que dans le Groupe S (respectivement: 64 % contre 91 %; différence des moyennes: 27 %; intervalle de confiance à 95 % [IC]: 13 à 41; P < 0,001). Le grade médian de la toux à l’extubation a été également plus faible dans le Groupe D. La tension artérielle et la FC moyennes ont été élevées dans le Groupe S pendant l’extubation trachéale alors que dans le Groupe D, elles ont été semblables aux valeurs de référence. Il n’y a pas eu de différence concernant la FR entre les deux groupes dans l’ensemble de l’étude. Un petit retard à l’extubation a été observé dans le Groupe D (3 minutes de plus que pour le Groupe S; IC à 95 %: 2 à 4; P < 0,001).
Conclusion
Comparativement à une perfusion de faible dose de remifentanil, seule, l’ajout d’une dose unique de dexmédétomidine (0,5 μg·kg−1) au cours de l’émergence d’une anesthésie par sévoflurane-remifentanil a efficacement atténué la toux et les changements hémodynamiques, et n’a pas exacerbé de dépression respiratoire après chirurgie de la thyroïde. Cette étude a été enregistrée sur le site www.clinicaltrials.gov sous le numéro: NCT01774305.
Similar content being viewed by others
Coughing during emergence from general anesthesia is a significant issue in the perioperative period as it may increase the risk of serious complications, including surgical bleeding, laryngospasm, and cardiovascular instability. This is particularly true for patients undergoing thyroid surgery where immediate postoperative bleeding can result in acute upper airway obstruction due to neck hematoma and increase the need for re-operation.1,2 Maintenance of a low-dose remifentanil infusion has been reported to decrease the incidence of coughing and induce a smooth emergence from general anesthesia.3 Several studies have shown that the optimal effect-site concentration (Ce) of remifentanil for cough suppression during emergence ranges from 1.5-2.9 μg·mL−1.4-7 Further increasing the dose of remifentanil can be effective in preventing coughing but may cause respiratory depression, delay emergence from anesthesia, and increase the incidence of postoperative nausea and vomiting.4,5
Dexmedetomidine is a selective α2-adrenoreceptor agonist that has dose-dependent sedative, analgesic, and sympatholytic properties.8,9 Dexmedetomidine produces sedation while sparing responsiveness to CO2, and thus, it has less effect on respiratory depression. Consequently, it has found utility and regulatory approval for use in bronchoscopic examinations10 and for weaning intensive care unit patients from mechanical ventilation.11 In addition, dexmedetomidine has been shown to reduce emergence agitation in children and adults after general anesthesia.12,13 Nevertheless, there has been limited investigation regarding the efficacy of a single dose of dexmedetomidine in preventing cough during emergence from anesthesia.
In this randomized double-blind placebo-controlled study, we hypothesized that a single dose of dexmedetomidine given just prior to emergence from general anesthesia in combination with an infusion of low-dose remifentanil would be more effective than remifentanil alone in preventing coughing and serious adverse events in patients undergoing thyroidectomy.
Methods
This prospective double-blind placebo-controlled trial was approved (protocol number: 3-2012-0142; September 2012) by the Institutional Review Board of Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. Following written informed consent, American Society of Anesthesiologists physical status I-II adults undergoing elective unilateral or total thyroidectomy were enrolled in our study. Exclusion criteria included the presence of an upper respiratory infection, asthma, anticipated difficult airway, hepatic dysfunction, poorly controlled hypertension (i.e., systolic blood pressure > 160 mmHg or diastolic blood pressure > 90 mmHg), or active coronary artery disease.
Patients were randomly assigned to either the dexmedetomidine group or the placebo group using a computer-based randomization program. The randomization result was kept sealed in an envelope, and only the nurse responsible for preparing the anesthetics was allowed to open the envelope and prepare the assigned drug. Each patient received an infusion of dexmedetomidine 0.5 μg·kg−1 (Group D) or normal saline (Group S) over ten minutes at the end of surgery.
Premedication consisted of glycopyrrolate 0.1 mg and midazolam 1-2 mg iv. Upon arrival in the operating room, standard vital sign monitors (pulse oximetry, electrocardiogram, and noninvasive blood pressure) were applied to the patients.14 Anesthesia was induced with propofol 1.5 mg·kg−1 and remifentanil (targeting a Ce of 1 ng·mL−1). The Ce of remifentanil (using Minto’s pharmacokinetic model)15,16 was maintained with a commercial target-controlled infusion (TCI) system (Orchestra® Base Primea; Fresenius Vial, Brezins, France). After confirmation of adequate muscle relaxation with the administration of rocuronium 0.6 mg·kg−1 iv, an endotracheal tube (ETT) with an internal diameter of 6.5 mm (female) or 7.5 mm (male) was inserted into the trachea. Topical lidocaine was not applied to the trachea. The ETT cuff pressure was maintained at 20-25 cm H2O. Mechanical ventilation using a tidal volume of 8 mL·kg−1 (ideal body weight) was adjusted to maintain the end-tidal CO2 partial pressure (ETCO2) at 35-40 mmHg in 50% O2/air. During surgery, expired sevoflurane was maintained at 2-2.5% with the remifentanil TCI adjusted to 1.5-4.0 ng·mL−1 to maintain heart rate (HR) and mean arterial pressure (MAP) within 20% of preoperative baseline values.
With the beginning of subcutaneous wound closure, the inhaled sevoflurane was titrated to 1%; the Ce of remifentanil was titrated to 1 ng·mL−1; the dexmedetomidine (or normal saline) was infused for ten minutes, and ketorolac 60 mg iv and ondansetron 4 mg iv were administered. Remifentanil was maintained at 1 ng·mL−1 until extubation in both groups. After completion of skin closure, sevoflurane was discontinued and 100% oxygen was administered at 6 L·min−1 until the time of extubation. Confirmation of neuromuscular function (using double burst stimulation monitoring) was ensured after neostigmine and glycopyrrolate administration. The ETCO2 was then maintained at 35-45 mmHg with manual ventilation. The patients were stimulated gently with intermittent verbal requests to open their eyes and with a light touch to the shoulder at 15-sec intervals. After eye opening in response to stimuli and recovery of adequate spontaneous ventilation, extubation was performed. Immediately after extubation, remifentanil was discontinued and 100% oxygen was given via a face mask for five minutes. All patients were then transferred to the postanesthetic care unit (PACU).
The primary endpoints were the incidence and severity of coughing from the time that sevoflurane was discontinued until five minutes after extubation. The study anesthesiologist, who was blinded to patient treatment group and did not participate in the anesthesia delivery, assessed and graded the severity and occurrence of coughing on a four-point scale: 0 = no cough; 1 = single cough; 2 = more than one episode of non-sustained cough; 3 = sustained and repetitive cough with head lift.17
The anesthesiologist blinded to group assignment also recorded the time from discontinuation of sevoflurane to first eye opening and extubation.
The respiratory rate (RR), MAP, and HR were recorded before anesthesia induction and dexmedetomidine/normal saline infusion, immediately after completion of dexmedetomidine/normal saline infusion, at first eye opening, during extubation, and every ten minutes in the PACU. In addition, the patients’ level of sedation was assessed on the Ramsay sedation scale18 (1 = the patient is anxious and restless or agitated, or both; 2 = the patient is cooperative, tranquil, and oriented; 3 = the patient responds to commands only; 4 = the patient exhibits a brisk response to a loud auditory stimuli or a light glabellar tap; 5 = the patient exhibits a sluggish response to a loud auditory stimulus or a light glabellar tap; and 6 = the patient exhibits no response). We also recorded length of stay (LOS) in the PACU and any adverse events, including hypertension (as defined previously), hypotension (systolic blood pressure < 90 mmHg), bradycardia (HR < 60 beats·min−1), oxygen desaturation (< 90%), breath-holding spells (apnea > 15 sec or apnea with desaturation), laryngospasm, re-exploration of operation site for hematoma, or postoperative nausea and vomiting. The attending anesthesiologist also recorded a numerical pain score (0-10; 0 = no pain, 10 = worst pain). Any adverse events were managed by an attending anesthesiologist according to institutional standards.
Statistical analysis
Calculation of sample size was based on the incidence of cough. In a previous study, the estimated incidence of cough during emergence from sevoflurane-remifentanil anesthesia with remifentanil TCI 1 ng·mL−1 was 63%.4 Assuming that the dexmedetomidine infusion reduces the incidence of cough by 50% and an α of 0.05, 64 patients would be required in each treatment group (assuming a power of 0.80). Anticipating a study drop-out rate of 10%, we included 71 patients per group.
All values were expressed as number of patients (%), mean (SD) or median value [interquartile range; IQR]. Data were analyzed for normally distributed continuous variables using the Student’s t test and for non-normally distributed continuous variables using the Mann-Whitney test. Discrete variables between the groups were compared using a Fisher’s exact test. Repeated measurements were analysed using linear mixed models with a Bonferroni correction. All reported P values are two-sided. Statistical analyses were performed using SAS® software 9.3 (SAS, Inc., Cary, NC, USA) and PASW statistics 20.0 Windows (SPSS, Chicago, IL, USA).
Results
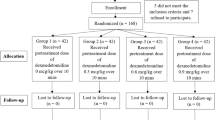
Among 142 patients enrolled in this study during September 2012 to May 2013, one patient in Group D did not complete the study due to an extended operation that included radical neck dissection (Fig. 1).
The two groups were comparable with respect to patient demographics and intraoperative variables (Table 1). The overall incidence of coughing was lower in Group D than in Group S (45 [64%] vs 65 [91%], respectively; mean difference 27%; 95% confidence interval [CI] 13 to 41; P < 0.001) (Table 2). In addition, the median values of cough grade were lower in Group D patients, both while the patients were still intubated and at extubation. Severe coughing examined as a post hoc secondary outcome was also lower in Group D.
The time to awakening and extubation were longer in Group D than in Group S (Table 3). A small delay (3 minutes longer than Group S) in extubation was observed in Group D (95% CI 2 to 4 min), and the Ramsay score at extubation was higher in Group D than in Group S. Nevertheless, the PACU LOS did not differ between the two groups.
The MAP and HR during tracheal extubation were higher in Group S, but they remained at the baseline values in Group D (Fig. 2). Group D also had a lower overall MAP and HR during recovery from anesthesia in the PACU. Nevertheless, at the time of discharge from the PACU, there were no differences in these hemodynamic variables between the two groups.
Hemodynamic changes. T0, baseline; T1, before the infusion of dexmedetomidine; T2, immediately after the infusion of dexmedetomidine; T3, eye opening spontaneously by light stimulation during emergence; T4, at extubation; T5, five minutes after postanesthesia care unit (PACU) admission; T6, at the discharge from PACU. Group D = treat with dexmedetomidine; Group S = treat with normal saline. *P < 0.05 compared with placebo group (Bonferroni corrected). † P < 0.05 compared with baseline in each group (Bonferroni corrected). Error bar showing standard deviation
Oxygen desaturation was not seen in either group, and the respiratory rate after extubation did not differ between the two groups. During the infusion of dexmedetomidine, bradycardia (HR < 60 beats·min−1) was noted in 42 patients (60%) without hypotension. Bradycardia resolved spontaneously in most of these patients after completion of the dexmedetomidine infusion. One patient in Group D had severe bradycardia (HR < 40 beats·min−1) that was treated with ephedrine 4 mg iv. There was a 1.4% incidence of re-exploration of the operation site for hematoma (one case in each group).
Discussion
In this randomized double-blind placebo-controlled study of patients undergoing general anesthesia for thyroidectomy, the addition of a single dose of dexmedetomidine (0.5 μg·kg−1) to a low-dose remifentanil infusion resulted in effective suppression of cough during emergence from a sevoflurane-based anesthetic. In addition, this drug combination did not appear to exacerbate respiratory depression in patients. The dexmedetomidine also blunted the cardiovascular response to tracheal extubation without increasing the incidence of serious adverse events. Nevertheless, dexmedetomidine did delay awakening and tracheal extubation, but this did not equate to any increased duration of PACU LOS.
Various modalities have been studied to suppress coughing on emergence, such as administration of intravenous lidocaine at the end of the surgery, topical lidocaine administration to the airway just prior to intubation, and filling the tracheal cuff with lidocaine.19 Intravenous administration of opioids are common for preventing cough during emergence. A continuous infusion of remifentanil during emergence from balanced anesthesia has previously been described for cough suppression because of the convenience and rapid decline of blood levels after discontinuation of remifentanil.20 Although high-dose remifentanil is highly effective for cough suppression, it may result in undesirable events, such as delayed awakening, increased postoperative nausea and vomiting, and prolonged PACU LOS.4,5 Remifentanil infusion at 2 ng·mL−1 via TCI depressed spontaneous respirations5 and was estimated as an effective concentration (EC95) for preventing cough during emergence from anesthesia.7 Thus, combination (with dexmedetomidine) therapy might be desirable for preventing cough as an alternate to increasing the concentration of remifentanil. Dexmedetomidine has analgesic and sedative effects without respiratory depression, and intraoperative infusions of dexmedetomidine have previously been shown to allow for a smooth emergence from anesthesia by attenuating agitation, cough, and hemodynamic changes in children.21 A recent study in adults after nasal surgery showed that an intraoperative dexmedetomidine infusion allowed for a smooth emergence from anesthesia by suppressing agitation.12 In that study, an intraoperative infusion of dexmedetomidine alone did not reduce the severity of cough and the median value of cough grade during emergence was still high.
In our study, additional dexmedetomidine not only reduced the incidence of any coughing but also reduced the severity of coughing in the presence of tracheal intubation and at extubation. In particular, dexmedetomidine decreased the median cough grade in tracheally intubated patients (Group D = 0 vs Group S = 1). This might have been due to deeper sedation rather than a specific antitussive effect, as dexmedetomidine has not previously been should to have an antitussive effect.22 Administration of a single dose of dexmedetomidine before the end of surgery is an easy and straightforward way to suppress coughing when compared with intraoperative infusion.
The addition of a single dose of dexmedetomidine to the remifentanil did not result in further respiratory depression, although this combination did induce a deeper level of sedation. Dexmedetomidine combined with remifentanil delayed the time to awakening and extubation; this is contrary to a previous report using dexmedetomidine alone at the same dose of 0.5 μg·kg−1.23 Nevertheless, a single dose of dexmedetomidine did not prolong the PACU LOS in accordance with a previous study of intraoperative dexmedetomidine infusion.12
The incidence of coughing in our study was relatively high (64% in Group D and 91% in Group P, respectively) compared with previous studies (up to 74%, even in a placebo control group) in patients after thyroidectomy under sevoflurane and remifentanil.4,24,25 The difference might be due to the characteristics of the patients in our study, the intensity of tactile stimuli during emergence from anesthesia, and the investigator’s technique during extubation. Furthermore, it might be caused by an overly sensitive evaluation of coughing because we considered small movements of the head at extubation as coughing. In support of this reasoning, the incidence of severe cough and the grade of cough were not excessively high.
A single dose of dexmedetomidine, despite its inherent sympatholytic effects,26 provided stable hemodynamics during emergence from anesthesia by suppressing elevation of blood pressure and tachycardia in accordance with previous studies.27 We administered the dexmedetomidine bolus (0.5 μg·kg−1) over ten minutes as rapid administration can produce bradycardia, tachycardia, and hypertension.9 Bradycardia did occur during dexmedetomidine infusion, but the MAP remained stable. We observed a similar hemodynamic change in a prior study when dexmedetomidine 1.0 μg·kg−1 was infused over ten minutes.28 After the infusion of dexmedetomidine was complete, HR recovered spontaneously, except in one patient who required treatment with ephedrine 4 mg iv. In our study, emergence from anesthesia and administration of glycopyrrolate along with an anticholinesterase drug might have prevented any significant decrease in HR. In addition, in consideration of the potential negative chronotropic activity of dexmedetomidine, some authors have reported sudden cardiac arrest after loading with dexmedetomidine.29 Indeed, in the PACU, dexmedetomidine appeared to lower MAP and HR compared with baseline values; however, at the time of discharge from the PACU, there were no differences between the two treatment groups.
There were some limitations to this study. For example, the study anesthesiologist who assessed the coughing could have been unblinded to the group assignment based on the patients’ hemodynamic changes. This could potentially have biased the assessment of cough.
In conclusion, the addition of a single dose of dexmedetomidine to a low-dose infusion of remifentanil during emergence from sevoflurane-remifentanil anesthesia was effective in attenuating cough without further respiratory depression after thyroid surgery. Furthermore, dexmedetomidine maintained hemodynamic stability during emergence from anesthesia. Nevertheless, delayed awakening might be associated with dexmedetomidine plus a low-dose infusion of remifentanil.
References
Lee HS, Lee BJ, Kim SW, et al. Patterns of post-thyroidectomy hemorrhage. Clin Exp Otorhinolaryngol 2009; 2: 72-7.
Harding J, Sebag F, Sierra M, Palazzo FF, Henry JF. Thyroid surgery: postoperative hematoma—prevention and treatment. Langenbecks Arch Surg 2006; 391: 169-73.
Aouad MT, Al-Alami AA, Nasr VG, Souki FG, Zbeidy RA, Siddik-Sayyid SM. The effect of low-dose remifentanil on responses to the endotracheal tube during emergence from general anesthesia. Anesth Analg 2009; 108: 1157-60.
Jun NH, Lee JW, Song JW, Koh JC, Park WS, Shim YH. Optimal effect-site concentration of remifentanil for preventing cough during emergence from sevoflurane-remifentanil anaesthesia. Anaesthesia 2010; 65: 930-5.
Chang CH, Lee JW, Choi JR, Shim YH. Effect-site concentration of remifentanil to prevent cough after laryngomicrosurgery. Laryngoscope 2013; 123: 3105-9.
Choi EM, Park WK, Choi SH, Soh S, Lee JR. Smooth emergence in men undergoing nasal surgery: the effect site concentration of remifentanil for preventing cough after sevoflurane-balanced anaesthesia. Acta Anaesthesiol Scand 2012; 56: 498-503.
Lee B, Lee JR, Na S. Targeting smooth emergence: the effect site concentration of remifentanil for preventing cough during emergence during propofol-remifentanil anaesthesia for thyroid surgery. Br J Anaesth 2009; 102: 775-8.
Gerlach AT, Dasta JF. Dexmedetomidine: an updated review. Ann Pharmacother 2007; 41: 245-52.
Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs 2000; 59: 263-8 discussion 269-70.
Shukry M, Miller JA. Update on dexmedetomidine: use in nonintubated patients requiring sedation for surgical procedures. Ther Clin Risk Manag 2010; 6: 111-21.
Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J Intensive Care Med 2003; 18: 29-41.
Kim SY, Kim JM, Lee JH, Song BM, Koo BN. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth 2013; 111: 222-8.
Lili X, Jianjun S, Haiyan Z. The application of dexmedetomidine in children undergoing vitreoretinal surgery. J Anesth 2012; 26: 556-61.
Merchant R, Chartrand D, Dain S, et al. Guidelines to the practice of anesthesia revised edition 2013. Can J Anaesth 2013; 60: 60-84.
Minto CF, Schnider TW, Egan TD, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology 1997; 86: 10-23.
Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanil. II. Model application. Anesthesiology 1997; 86: 24-33.
Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth Analg 2004; 99: 1253-7.
Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J 1974; 2: 656-9.
Shroff PP, Patil V. Efficacy of cuff inflation media to prevent postintubation-related emergence phenomenon: air, saline and alkalinized lignocaine. Eur J Anaesthesiol 2009; 26: 458-62.
Egan TD, Minto CF, Hermann DJ, Barr J, Muir KT, Shafer SL. Remifentanil versus alfentanil: comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology 1996; 84: 821-33.
Patel A, Davidson M, Tran MC, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg 2010; 111: 1004-10.
Ryu JH, Lee SW, Lee JH, Lee EH, Do SH, Kim CS. Randomized double-blind study of remifentanil and dexmedetomidine for flexible bronchoscopy. Br J Anaesth 2012; 108: 503-11.
Guler G, Akin A, Tosun Z, Eskitascoglu E, Mizrak A, Boyaci A. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand 2005; 49: 1088-91.
Kim H, Choi SH, Choi YS, Lee JH, Kim NO, Lee JR. Comparison of the antitussive effect of remifentanil during recovery from propofol and sevoflurane anaesthesia. Anaesthesia 2012; 67: 765-70.
Lee JH, Koo BN, Jeong JJ, Kim HS, Lee JR. Differential effects of lidocaine and remifentanil on response to the tracheal tube during emergence from general anaesthesia. Br J Anaesth 2011; 106: 410-5.
Talke P, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg 1997; 85: 1136-42.
Guler G, Akin A, Tosun Z, Ors S, Esmaoglu A, Boyaci A. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth 2005; 15: 762-6.
Wang T, Ge S, Xiong W, Zhou P, Cang J, Xue Z. Effects of different loading doses of dexmedetomidine on bispectral index under stepwise propofol target-controlled infusion. Pharmacology 2013; 91: 1-6.
Bharati S, Pal A, Biswas C, Biswas R. Incidence of cardiac arrest increases with the indiscriminate use of dexmedetomidine: a case series and review of published case reports. Acta Anaesthesiol Taiwan 2011; 49: 165-7.
Acknowledgements
The authors sincerely thank Hanna Yoo, PhD for expert statistical analysis.
Financial support and sponsorship
No funding.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Yon Hee Shim supervised the conception and design of the study and wrote the manuscript. Jeong Soo Lee and Seung Ho Choi participated in conducting the experiments and drafting the manuscript. Jeong Soo Lee, Seung Ho Choi, Young Ran Kang, and Yunhee Kim contributed to the data collection and analysis. Young Ran Kang and Yunhee Kim contributed to the study design. All authors contributed to the final manuscript.
Rights and permissions
About this article
Cite this article
Lee, J.S., Choi, S.H., Kang, Y.R. et al. Efficacy of a single dose of dexmedetomidine for cough suppression during anesthetic emergence: a randomized controlled trial. Can J Anesth/J Can Anesth 62, 392–398 (2015). https://doi.org/10.1007/s12630-014-0295-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0295-6