Abstract
Purpose
Variability in drug responses could result from both genetic and environmental factors. Thus, drug effect could depend on geographic location, although regional variation is not generally acknowledged as a basis for stratification. There is evidence that the pharmacokinetic set developed in a European population for the target-controlled infusion (TCI) of propofol does not apply in Chinese patients; however, we are not aware of previous studies comparing the estimated concentration-bispectral index (BIS) response of Caucasian patients in Europe with that of Chinese patients in China.
Methods
The DiprifusorTM TCI pump, incorporating the pharmacokinetic model proposed by Marsh et al., was applied to 30 Caucasian patients in Austria and 30 Chinese patients in China. The estimated plasma concentration (Cp) of propofol for the two groups was set at 1 μg·mL−1 and increased by 1 μg·mL−1 every minute to gradually reach 5 μg·mL−1 after 5 min. The BIS values were fitted against the estimated Cp and the predicted effect-site concentration (Ce) in a sigmoid Emax model.
Results
The sigmoid Emax curves were shifted significantly to the left in the Chinese group compared with the Austrian group. After 5 min, the BIS value in the Chinese group was lower than in the Austrian group (mean ± standard deviation [SD], 47.2 ± 3.6 vs 63.6 ± 5.4, respectively; P = 0.0006). The estimated Cp at loss of consciousness (LOC), predicted Ce at LOC, and time to LOC, were lower in the Chinese group than in the Austrian group (3.3 ± 0.8 μg·mL−1, 1.6 ± 0.4 μg·mL−1, 2.8 ± 0.6 min, respectively, vs 4.6 ± 2.8 μg·mL−1, 2.4 ± 1.5 μg·mL−1, 3.9 ± 0.5 min, respectively; P < 0.0001).
Conclusion
When propofol is given using the same TCI protocol, Chinese patients in China lost consciousness faster and at a lower estimated plasma concentration than Caucasians in Austria. Larger studies are needed to map geographically appropriate TCI infusion models.
Résumé
Objectif
La variabilité de la réponse aux médicaments pourrait être due à des facteurs génétiques aussi bien qu’environnementaux. Dès lors, l’effet d’un médicament pourrait dépendre de l’emplacement géographique, bien que la variation géographique ne soit en général pas prise en compte comme base pour la stratification. Des données probantes soutiennent que les données pharmacocinétiques recueillies chez une population européenne pour une anesthésie intraveineuse à objectif de concentration (AIVOC) au propofol se s’applique pas aux patients chinois; cependant, nous n’avons pas trouvé d’études antérieures comparant l’indice bispectral (BIS) en fonction de la concentration estimée de patients d’origine caucasienne en Europe à celle de patients chinois en Chine.
Méthode
La pompe d’AIVOC DiprifusorTM intégrant le modèle pharmacocinétique proposé par Marsh et coll. a été appliquée à 30 patients d’origine caucasienne en Autriche et 30 patients chinois en Chine. La concentration plasmatique (Cp) estimée de propofol pour les deux groupes a été fixée à 1 μg·mL−1 et augmentée de 1 μg·mL−1 par minute afin d’atteindre progressivement 5 μg·mL−1 après 5 min. On a mis en graphique les valeurs de BIS ont été en fonction de la Cp estimée et de la concentration au site effecteur prévue (Ce) dans un modèle Emax sigmoïde.
Résultats
Les courbes Emax sigmoïdes étaient significativement plus à gauche pour le groupe chinois que dans le groupe autrichien. Après 5 min, la valeur de BIS du groupe chinois était plus basse que celle du groupe autrichien (moyenne ± écart type [ET], 47,2 ± 3,6 vs. 63,6 ± 5,4, respectivement; P = 0,0006). La Cp estimée lors de la perte de conscience (PC), la Ce prévue lors de la PC, et le temps jusqu’à PC étaient plus bas dans le groupe chinois que dans le groupe autrichien (3,3 ± 0,8 μg·mL−1, 1,6 ± 0,4 μg·mL−1, 2,8 ± 0,6 min, respectivement, vs. 4,6 ± 2,8 μg·mL−1, 2,4 ± 1,5 μg·mL−1, 3,9 ± 0,5 min, respectivement; P < 0,0001).
Conclusion
Lorsque administré selon le même protocole d’AIVOC, le propofol fait perdre conscience plus rapidement et à une concentration plasmatique estimée plus basse chez les Chinois en Chine que chez les personnes d’origine caucasienne en Autriche. Des études d’envergure plus importante sont nécessaires afin de créer des modèles de perfusion d’AIVOC géographiquement adaptés.
Similar content being viewed by others
Geographic variation in drug response could arise from genetics and a variety of environmental factors that often are difficult to define or quantify. Despite evidence that propofol requirements and recovery times vary between patients living in different geographic locations,1,2 regional variation is still not acknowledged as a factor that could influence drug effect. This has been a relatively neglected area of investigation. Despite the fact that authorities in some countries, e.g., Japan, Korea, and People’s Republic of China, require information on local populations before drug registration, drugs remain licensed under the same recommended dose for patients in different geographic locations. The local licensing authorities accept data from a limited number of European or North American Caucasian subjects and do not demand specific information on precise dosage efficacy and toxicity from patients in their geographic location.
The bispectral index (BIS) is a processed electroencephalographic (EEG) parameter derived from an empirical database using a complex proprietary algorithm that combines three subparameters into a single metric.3 It quantifies EEG response to anesthetics. The DiprifusorTM (AstraZeneca Pharmaceuticals, Macclesfield, UK) target-controlled infusion (TCI) pump uses a pharmacokinetic model to achieve and maintain a set propofol plasma concentration4; however, plasma is not the site of drug effect. Thus, the Diprifusor displays both the estimated plasma concentration (Cp) and the predicted effect-site concentration (Ce).4
There are studies wherein estimated concentrations of propofol have been examined at peak BIS effect using a TCI technique in Chinese populations.5,6 Other studies using logistic regression sigmoid Emax curves individually assessed the propofol estimated effective concentration (EC50) at which 50% of Caucasian7 or Chinese patients8 did not respond to skin incision. We are not aware of previous studies directly comparing the performance of the widely used Diprifusor system, which incorporates the commonly used Marsh pharmacokinetic model,9 between Chinese patients in China and Caucasian patients in Europe. There are data in the literature showing that estimated concentrations according to the Marsh pharmacokinetic model did not accurately reflect measured blood concentrations in the Chinese population,10 or in other populations,11 but no model leads to accurate predictions. Thus, we assumed that the Marsh pharmacokinetic model might not apply to either the Chinese or the Austrian group, even if it was developed in a European population. The propofol effect was measured using the widely available BIS monitor, which was developed primarily in a North American population.3 Thus, we sought to examine whether the administration of propofol using a TCI technique based on the popular Marsh pharmacokinetic model,9 and thereby providing the same estimated concentration to both groups, resulted in the same dynamic end points, such as loss of consciousness (LOC), in patients with different geographic origins, i.e., Chinese patients in China and Caucasian patients in Austria. The purpose of our study was to compare the estimated plasma concentration-response (Cp-BIS) and the predicted effect site concentration-response (Ce-BIS) in a group of Caucasian patients in Austria vs a group of Han Chinese patients in China.
Methods
Our study was registered at the European Clinical Trials Database, EudraCT (trial registration number: 2009-015582-31). Our report was prepared in conformity with the guidelines of the Consolidated Standards of Reporting Trials (CONSORT) statement.12 A prospective controlled study was conducted at the Medical University of Graz, Austria and the Zhe Jiang University hospitals, People’s Republic of China from September 2008 to March 2010. After approval by the ethics committees, all patients who agreed to participate in the study gave written informed consent. As health differences were a major concern, we delineated the health status between groups by including only those patients who were American Society of Anesthesiologists (ASA) classification I and II with body mass index 18-26 kg·m−2 and aged 30-50 yr. We excluded patients with alcohol, tobacco, or substance abuse, or medical conditions that might affect their level of consciousness, such as stroke, stupor, or encephalopathies. Thirty consecutive (15 male/15 female) second-generation Han Chinese patients, who were living in China for at least ten years and scheduled to undergo general anesthesia for orthopedic or general surgery, were recruited at the Zhe Jiang University hospitals. The Chinese patients were matched with 30 second-generation Caucasian patients who were living in Austria for at least ten years, met the same sex, ASA classification, and approximate age and weight criteria, and were recruited at the Medical University of Graz, Austria. The Caucasian patients were classified as members of the Indo-European white race from supposed origin in the Caucasus.
A BIS Quatro Sensor (Aspect Medical Systems, Newton, MA, USA) was placed on each patient’s forehead according to manufacturer’s guidelines and connected to a BIS-XP monitor. The raw EEG signal was band-pass filtered to 2-70 Hz and processed in real time using version 3.4 of the BIS algorithm. In addition, the BIS monitor displayed the electromyographic (EMG) activity calculated in decibels (dB) in the 70-110 Hz frequency band. The BIS recordings were started after verifying a signal quality index > 95%, electrode impedance < 5 kΩ, and EMG values < 35 dB.
The patients received no premedication prior to the study. Propofol was obtained from the same AstraZeneca Pharmaceuticals European production line, and the same study protocol was strictly implemented in both countries. A propofol DiprifusorTM (software version 2) was used that incorporated the Marsh pharmacokinetic model.9 A target-controlled infusion was started after the patients’ anthropometric data were entered. In all patients, the Diprifusor Cp was set to reach a target of 5 μg·mL−1 gradually over five minutes by initially setting the target at 1 μg·mL−1 and increasing the target by 1 μg·mL−1 increments every minute. Thus, the estimated Cp variation with time was the same for all patients. The exit rate constant out of the effect site (keo) was set at 0.26 min−1 to obtain effect-site concentrations (Ce). After 5 min, the estimated Cp was maintained at 5 μg·mL−1 with no further changes. To eliminate differences in the observers’ approaches to LOC, we used a consistent set of identical verbal commands in the respective languages. With a gentle tap on the shoulder every ten seconds, patients were asked whether they were still awake until a score of one was reached on the Observer’s Assessment of Alertness and Sedation (OAA/S) Scale, i.e., no eyelash reflex and no response to verbal commands, which defined LOC.5 The patients’ lungs were ventilated via a facemask. Digitized BIS values, estimated Cp, and predicted Ce were collected continuously and stored on a laptop computer for the duration of the study. Stable BIS values that showed no further decline and remained within ± 5 of the previous BIS value were considered an indicator of a steady state propofol concentration. A remifentanil infusion of 0.1-0.3 μg·kg−1·min−1 was then started, and rocuronium 600 μg·kg−1 was administered for tracheal intubation, which marked the end of our study recording period. The BIS values showing artifacts, which are very clearly displayed as such in the off-line format, were identified and eliminated in the off-line analysis. Patients were warmed using a forced hot-air blanket, and mean arterial pressure and heart rate were recorded continuously.
Statistical analysis
Using the NONMEM Scientist® program (MicroMath Scientific Software Inc., Saint Louis, MO, USA), propofol estimated Cp-BIS and predicted Ce-BIS relationships were modelled using a non-linear regression analysis (NLR) via a non-linear mixed effects modelling (NONMEM) sigmoid Emax pharmacokinetic-pharmacodynamic model.13 The goodness of fit of each patient was determined using Akaike’s information criterion.14 Each patient’s Cp-BIS and Ce-BIS data points were fitted to a sigmoid Emax mathematical model of the nature:
where Ebaseline is the BIS value before any propofol administration (BIS = 100); Emax is the BIS value at maximal propofol effect, i.e., when steady state Cp = 5 μg·mL−1 is reached; C50 is the estimated concentration when 50% of Emax is obtained; and γ is a Hill coefficient sigmoidicity factor, i.e., the slope of the sigmoid curve. In all patients over the five-minute period, we obtained a consistent set of BIS vs estimated Cp data points on the sigmoid curves, which were used to create the sigmoid curves of the two geographical locations.
Our primary outcome measurement was Emax (lowest BIS value at estimated Cp = 5 μg·mL−1). We performed an interim power analysis Student’s t test (α = 0.05) based on the first ten Austrian pilot patients who were included in the final analysis, which indicated a mean BIS Emax of 54.8 ± 6.4 compared with 43.2 ± 2.6 for ten Chinese patients. This result showed that a group size of 25 patients would be required to reveal a statistically significant difference between the two groups with > 90% power.
We used repeated measures analysis of variance (ANOVA) to compare parameter differences over time (group and time factors), and we used Dunnett’s two-sided multiple comparison post hoc test to compare BIS values at different propofol estimated plasma concentrations. Data were expressed as means ± SD, with P < 0.05 considered statistically significant.
Results
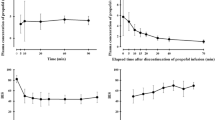
Patients’ demographic data are presented in Table 1. Compared with the Austrian group, the Cp-BIS (Fig. 1) and Ce-BIS (Fig. 2) sigmoid Emax curves were shifted significantly to the left in the Chinese group. The lowest BIS value at the propofol estimated Cp of 5 μg·mL−1 was less in the Chinese group than in the Austrian group (47.2 ± 3.6 vs 63.6 ± 5.4, respectively; P = 0.0006).
The propofol estimated Cp at LOC, the predicted Ce at LOC, and time to LOC were significantly less in the Chinese group than in the Austrian group; however patients of both groups lost consciousness at similar BIS values (Table 2). Two-way ANOVA (group x time) revealed differences (P < 0.0001) in BIS values over time. Dunnett’s two-sided multiple comparison post hoc test revealed differences between the two groups (P < 0.001) in BIS values starting from the propofol estimated Cp = 2.0 μg mL−1.
Before induction, the mean arterial pressure was greater in the Chinese patients than in the Austrian patients (99 ± 15 mmHg vs 90 ± 14 mmHg); however, after induction, there was a similar decrease with time in both groups to 90 ± 15 mmHg vs 80 ± 15 mmHg, respectively (two-way ANOVA). Heart rate was almost identical in the Chinese and Austrian groups both before and after induction (77 ± 16 beats·min−1 vs 76 ± 12 beats·min−1, respectively, and 74 ± 14 beats·min−1 vs 75 ± 10 beats·min−1, respectively).
Discussion
Using a similar estimated Cp based on the Marsh pharmacokinetic model, which incorporates patients’ age, weight, and height, we demonstrated that Chinese patients lost consciousness 1.1 min earlier than their Austrian counterparts. This result corresponds with an estimated propofol Cp that was 0.8 μg·mL−1 lower. Interestingly, patients of both groups lost consciousness at similar BIS values. This could be interpreted either as Chinese patients responding more to the same estimated propofol concentration as their Austrian counterparts (a pharmacodynamic explanation), or as Chinese individuals achieving a greater propofol plasma concentration following the same Diprifusor infusion (a pharmacokinetic explanation), or a combination of both factors. This issue could be resolved only by measurement of propofol blood concentrations. Irrespective of the true explanation, the Diprifusor concentration settings should be reduced in Chinese patients. The pharmacokinetic model used to drive the Diprifusor was probably erroneous in both populations, because it missed the target BIS value of 40 in both groups (BIS 47 in Chinese patients and BIS 64 in Austrian patients).
We demonstrated a significant difference in both the propofol estimated Cp-BIS as well as the propofol predicted Ce-BIS response relationships between the Austrian and Chinese patients. The Ce-BIS is probably the more clinically relevant of these, because the site of action of propofol on BIS, an EEG-derived parameter, is the brain, not the plasma. Our results seem to concur with the results of a previous study in which recovery time from anesthesia that was maintained at similar propofol infusion rates was shorter in Caucasian than in Chinese patients.1 Our group also demonstrated a geographic difference concerning a neuromuscular blocking agent, as the duration of action of rocuronium was significantly longer in Han Chinese patients in China than in Caucasians in Austria.15
On the other hand, our study results do not concur with other individual studies that failed to reveal or precisely quantify differences between Caucasian and Chinese populations.7,8 Two separate studies conducted by the same team implementing the same study design reported almost identical results for Caucasians in the United Kingdom7 and Chinese patients in Hong Kong.8 The estimated EC50 at LOC was 2.8 μg·mL−1 in Caucasian patients in the United Kingdom7 and 2.7 μg·mL−1 in Chinese patients in Hong Kong.8 However, this could also indicate that a pharmacokinetic explanation seems to be a more plausible explanation rather than a pharmacodynamic one.
Despite the fact that patients in a single geographic region share certain traits, both genetic and environmental, in today’s multicultural world, populations from different or even mixed ethnic origins could be living in distant geographic locations. Thus, we tried to identify two ethnic populations that share the same factors that could affect propofol distribution, such as diet, pollution, physical exercise, body composition, fat distribution, and skeletal muscle fibre type proportion. Our study was an attempt to evaluate the “collective effect” of these combined ethnic/environmental variables. It was not the aim of our study to determine which variables might have contributed to our findings. That being said, the differences between the Chinese and Caucasian patients we encountered in our study are likely to be multifactorial.
A possible explanation of our results is the degree of plasma protein binding in both populations. Only the unbound free fraction can exert a pharmacological effect. However changes in plasma protein binding were shown to have very little clinical relevance,16–18 except for a small group of drugs, such as propofol, that are extensively protein bound (>96%) and have a very large volume of distribution.19,20 This was clearly demonstrated when sudden cardiopulmonary bypass hemodilution resulted in a twofold increase in the unbound propofol with an immediate decrease in BIS values, despite propofol total plasma concentration remaining unchanged.21 Compared with the Caucasians, the Chinese subjects were shown to have different plasma protein binding to drugs, as they possess lower α-1-acid glycoprotein plasma concentrations, a glycoprotein shown to accelerate recovery from drugs by increasing protein binding.22
The effect of propofol, with its high hepatic extraction ratio23 is expected to depend on hemodynamic changes during induction. In our study, hemodynamic parameters did not seem to play a significant role, as there were no significant differences between the two groups. However, cardiac output was not measured, and this factor cannot be ruled out to account for faster response in Chinese patients.24
Our study has limitations, as we did not confirm the actual propofol concentrations through blood samples. Furthermore, as we only assessed the Marsh pharmacokinetic model,9 it would have been interesting to see how other TCI models, such as the Schnider model,25 would describe both populations.
Finally, the significant differences in propofol BIS responses to similar infusion regimens would necessitate either a TCI pharmacokinetic model designed especially for a Chinese population or a change in target concentrations using the same model. Given that there is probably already considerable and often unpredictable variability even among individuals of the same ethnic origin, TCI propofol should be titrated to clinical effect.
In conclusion, we demonstrated significant differences between Han Chinese and Austrian Caucasians in BIS responses to similar propofol infusion regimens. Given our observed results of clear differences between the two populations, we recommend larger studies in different geographic locations to prepare a map of differential propofol TCI infusion models, in addition to those already established predominantly for the Caucasian population. Larger studies of propofol clinical pharmacology are warranted to explore all potential covariates that may contribute to our understanding of these differences, along with a thorough analysis of why these differences occur and in which ethnic groups these covariates are relevant.
References
Ortolani O, Conti A, Chan YK, Sie MY, Ong GS. Comparison of propofol consumption and recovery time in Caucasians from Italy, with Chinese, Malays and Indians from Malaysia. Anaesth Intensive Care 2004; 32: 250-5.
Ortolani O, Conti A, Ngumi ZW, et al. Ethnic differences in propofol and fentanyl response: a comparison among Caucasians, Kenyan Africans and Brazilians. Eur J Anaesthesiol 2004; 21: 314-9.
Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology 1998; 89: 980-1002.
White M, Schenkels MJ, Engbers FH, et al. Effect-site modelling of propofol using auditory evoked potentials. Br J Anaesth 1999; 82: 333-9.
Xu Z, Liu F, Yue Y, et al. C50 for propofol-remifentanil target-controlled infusion and bispectral index at loss of consciousness and response to painful stimulus in Chinese patients: a multicenter clinical trial. Anesth Analg 2009; 108: 478-83.
Zhang MZ, Yu Q, Huang YL, Wang SJ, Wang XR. A comparison between bispectral index analysis and auditory-evoked potentials for monitoring the time to peak effect to calculate the plasma effect site equilibration rate constant of propofol. Eur J Anaesthesiol 2007; 24: 876-81.
Milne SE, Troy A, Irwin MG, Kenny GN. Relationship between bispectral index, auditory evoked potential index and effect-site EC50 for propofol at two clinical end-points. Br J Anaesth 2003; 90: 127-31.
Irwin MG, Hui TW, Milne SE, Kenny GN. Propofol effective concentration 50 and its relationship to bispectral index. Anaesthesia 2002; 57: 242-8.
Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth 1991; 67: 41-8.
Li YH, Xu JH, Yang JJ, Tian J, Xu JG. Predictive performance of ‘Diprifusor’ TCI system in patients during upper abdominal surgery under propofol/fentanyl anesthesia. J Zhejiang Univ Sci B 2005; 6: 43-8.
Coetzee JF, Glen JB, Wium CA, Boshoff L. Pharmacokinetic model selection for target controlled infusions of propofol. Assessment of three parameter sets. Anesthesiology 1995; 82: 1328-45.
Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001; 357: 1191-4.
Minto CF, Schnider TW, Short TG, Gregg KM, Gentilini A, Shafer SL. Response surface model for anesthetic drug interactions. Anesthesiology 2000; 92: 1603-16.
Dahaba AA, Oettl K, von Klobucar F, Reibnegger G, List WF. End stage renal failure reduces central clearance and prolongs the elimination half life of remifentanil. Can J Anesth 2002; 49: 369-74.
Dahaba AA, Perelman SI, Moskowitz DM, et al. Geographic regional differences in rocuronium bromide dose-response relation and time course of action: an overlooked factor in determining recommended dosage. Anesthesiology 2006; 104: 950-3.
Benet LZ, Hoener BA. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther 2002; 71: 115-21.
Rolan PE. Plasma protein binding displacement interactions -why are they still regarded as clinically important? Br J Clin Pharmacol 1994; 37: 125-8.
MacKichan JJ. Protein binding drug displacement interactions fact or fiction? Clin Pharmacokinet 1989; 16: 65-73.
Hiraoka H, Yamamoto K, Okano N, Morita T, Goto F, Horiuchi R. Changes in drug plasma concentrations of an extensively bound and highly extracted drug, propofol, in response to altered plasma binding. Clin Pharmacol Ther 2004; 75: 324-30.
Mazoit JX, Samii K. Binding of propofol to blood components: implications for pharmacokinetics and for pharmacodynamics. Br J Clin Pharmacol 1999; 47: 35-42.
Takizawa E, Hiraoka H, Takizawa D, Goto F. Changes in the effect of propofol in response to altered plasma protein binding during normothermic cardiopulmonary bypass. Br J Anaesth 2006; 96: 179-85.
Zhou HH, Adedoyin A, Wilkinson GR. Differences in plasma binding of drugs between Caucasians and Chinese subjects. Clin Pharmacol Ther 1990; 48: 10-7.
Dahaba AA, von Klobucar F, Rehak PH, List WF. Total intravenous anesthesia with remifentanil, propofol and cisatracurium in end stage renal failure. Can J Anesth 1999; 46: 696-700.
Absalom AR, Mani V, De Smet T, Struys MM. Pharmacokinetic models for propofol—defining and illuminating the devil in the detail. Br J Anaesth 2009; 103: 26-37.
Schnider TW, Minto CF, Gambus PL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 1998; 88: 1170-82.
Acknowledgement
The authors sincerely thank Mr. Xiao-Xuan Fan and Ms. Zhu Jun for their great efforts and meticulous work in preparing the manuscript.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is accompanied by an editorial. Please see Can J Anesth 2011; 58(4).
Rights and permissions
About this article
Cite this article
Dahaba, A.A., Zhong, T., Lu, H.S. et al. Geographic differences in the target-controlled infusion estimated concentration of propofol: bispectral index response curves. Can J Anesth/J Can Anesth 58, 364–370 (2011). https://doi.org/10.1007/s12630-011-9453-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-011-9453-2