Abstract
Purpose
Although guidelines for difficult airway management have been published, the extent to which consultant anesthesiologists follow these guidelines has not been determined. The purpose of this study is to observe how consultant anesthesiologists manage a “cannot intubate, cannot ventilate” (CICV) scenario in a high-fidelity simulator and to evaluate whether a simulation teaching session improves their adherence to the American Society of Anesthesiologists (ASA) difficult airway algorithm.
Methods
With Ethics Board approval and informed consent, all staff anesthesiologists in a single tertiary care institution were invited to enrol in this study where they managed a simulated unanticipated CICV scenario in a high-fidelity simulator. The scenario involved a patient with a difficult airway whose trachea could not be intubated and where it was impossible to ventilate the patient’s lungs. Airway management options, including laryngeal mask airway, a fibreoptic bronchoscope, and a Glidescope® were available for use but scripted to fail. A percutaneous cricothyroidotomy was required to re-establish adequate ventilation. Following the scenario, there was a personalized one-hour video-assisted expert debriefing focusing on the ASA difficult airway guidelines and “hands-on” cricothyroidotomy teaching. The second scenario followed immediately with an identical CICV scenario. The content to either scenario was not revealed beforehand. Outcome measures included: 1) major deviations from the ASA difficult airway guidelines; 2) time to start cricothyroidotomy; and 3) time to achieve ventilation.
Results
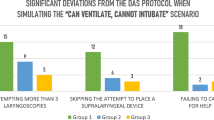
Thirty-eight anesthesiologists agreed to participate. The number of major deviations from the ASA algorithm was similar in the first and second sessions. These deviations included: multiple laryngoscopies (0 vs 2 pre-post; P = 0.49), use of fibreoptic bronchoscope (8 vs 7 pre-post; P = 1.0), bypass of laryngeal mask airway attempt (7 vs 13 pre-post; P = 0.19), and failure to call for anesthetic help (12 vs 8 pre-post; P = 0.43). However, more participants failed to call for surgical help in the second session (7 vs 16; P = 0.04). The times to start cricothyroidotomy and the times to achieve ventilation were significantly shorter in the second session (205.5 ± 61.3 sec vs 179.7 ± 65.1 sec; P = 0.01 and 356.9 ± 117.2 sec vs 269.4 ± 77.43 sec; P = 0.0002, respectively).
Conclusion
No substantial changes in airway management in a CICV scenario were observed after an intense one-hour personalized video-assisted airway-focused simulation debriefing session with an expert. It appears that multiple factors other than airway algorithms come into play in emergency airway decision-making processes, including one’s personal clinical experience with the many available airway devices.
Résumé
Objectif
Bien que les lignes directrices relatives à la prise en charge de l’intubation difficile aient été publiées, on ne sait pas dans quelle mesure les anesthésiologistes consultants s’y conforment. L’objectif de cette étude consiste à observer la manière dont les anesthésiologistes consultants gèrent une situation lors de laquelle l’intubation et la ventilation sont impossibles (« cannot intubate, cannot ventilate » - CICV) dans le cadre d’une simulation haute fidélité, ainsi que d’évaluer si la tenue d’une séance de formation par simulation accroît l’observance de l’algorithme de prise en charge de l’intubation difficile de l’American Society of Anesthesiologists (ASA).
Méthodes
Avec l’approbation du comité d’éthique et un consentement éclairé, tous les anesthésiologistes d’une seule institution de soins tertiaires ont été invités à participer à cette étude et à gérer un scénario CICV imprévu dans le cadre d’une simulation haute fidélité. Le scénario impliquait un cas d’intubation difficile chez un patient dont la trachée ne pouvait être intubée et dont les poumons ne pouvaient être ventilés. Des options de prise en charge des voies aériennes, qui incluaient un masque laryngé, un bronchoscope à fibres optiques et un Glidescope®, étaient disponibles, mais le scénario prévoyait leur échec. Une cricothyroïdotomie percutanée était nécessaire pour rétablir une ventilation adéquate. Au terme du scénario, les participants ont assisté à une séance d’évaluation dirigée par un expert, personnalisée et assistée par une vidéo d’une heure portant sur les lignes directrices de l’ASA pour la prise en charge de l’intubation difficile et la formation « pratique » en cricothyroïdotomie. Cette étape était immédiatement suivie d’un deuxième scénario CICV identique. Le contenu des deux scénarios n’était pas révélé à l’avance. Les indicateurs de résultat incluaient : 1) les déviations majeures aux lignes directrices de l’ASA pour la prise en charge de l’intubation difficile; 2) le délai nécessaire à la mise en œuvre de la cricothyroïdotomie; et 3) le délai nécessaire pour assurer la ventilation.
Résultats
Trente-huit anesthésiologistes ont accepté de participer à l’étude. Le nombre de déviations majeures à l’algorithme de l’ASA était identique lors de la première et de la deuxième séance. Ces déviations comprenaient: les laryngoscopies multiples (0 contre 3 avant-après; P = 0,49), l’utilisation d’un bronchoscope à fibres optiques (8 contre 7 avant-après; P = 1,0), l’omission de la tentative de pose du masque laryngé (7 contre 13 avant-après; P = 0,19) et le défaut de demander de l’aide sur le plan anesthésique (12 contre 8 avant-après; P = 0,43). Cependant, pendant la deuxième séance, un plus grand nombre de participants ont omis de demander de l’aide sur le plan chirurgical (7 contre 16; P = 0,04). Les délais nécessaires pour la mise en œuvre de la cricothyroïdotomie et pour assurer la ventilation étaient beaucoup plus courts pendant la deuxième séance (205,5 ± 61,3 sec. contre 179,7 ± 65,1 sec.; P = 0,01, et 356,9 ± 117,2 sec. contre 269,4 ± 77,43 sec.; P = 0,0002, respectivement).
Conclusion
Dans le cadre d’un scénario CICV, aucun changement important de la prise en charge des voies aériennes n’a été observé après une séance d’évaluation personnalisée assistée par vidéo, dirigée par un expert et portant sur les voies aériennes. Il semble que de nombreux facteurs, outre les algorithmes de prise en charge de l’intubation, entrent en jeu lors des processus de prise de décisions liées à l’intubation d’urgence, y compris l’expérience clinique personnelle avec les nombreux dispositifs disponibles pour les voies aériennes.
Similar content being viewed by others
Airway management is a fundamental anesthetic skill and responsibility. It is estimated that 100 to 700 real-life “cannot intubate, cannot ventilate” (CICV) events are managed in Canada every year.1 Despite the publication of various practice guidelines for the management of CICV,2-4 poor outcomes related to mismanagement of CICV situations, such as death and brain damage, still persist.5-10 Situational stress, low familiarity with other techniques of tracheal intubation other than direct laryngoscopy, and lack of adherence to published emergency airway algorithms are thought to be important reasons for this reality.1,3,7,8
Simulation has proven value in training management of emergency situations in anesthesia.11,12 High-fidelity simulation reproduces the conditions under which lifesaving decisions need to be made, adding psychological stress and time pressure, factors known to affect performance.13 The objective of this prospective controlled single-blinded study was to observe how consultant anesthesiologists currently manage a standard CICV scenario. We also sought to observe the effects on management of a second CICV scenario immediately following a one-hour debriefing session. We hypothesized that consultant anesthesiologists would deviate from American Society of Anesthesiologists (ASA) difficult airway algorithm before training and that simulation-based education would improve their adherence to guidelines.
Methods
After obtaining Research Ethics Board approval for the protocol and written informed consent, all consultant anesthesiologists from a single tertiary institution were approached for recruitment during April of 2008 to January of 2009.
Simulation setup and sessions
The simulation room included a fully monitored (ECG, NIBP, SpO2, ETCO2) high-fidelity mannequin (SimMan®; Laerdal Medical, Kent, UK) and basic airway devices (multiple size laryngoscope blades, endotracheal tubes, laryngeal mask airways, and a gum elastic bougie). A videolaryngoscope (Glidescope®) and/or the airway cart were brought in at request. The airway cart carried a fibreoptic bronchoscope (FOB) and the 4.0 mm Melker emergency cricothyroidotomy catheter set (C-TCCS-400; Cook Inc., Bloomington, IN, USA).
To familiarize the participants with managing the airway on the simulator, they were initially presented with a “cannot intubate, can ventilate” introductory scenario in which the use of any airway adjunct after induction of anesthesia would result in successful intubation and conclusion of the scenario. This introductory scenario was not used as part of the study. No debriefing with regard to the ASA algorithm occurred after the introductory scenario.
The participants were then presented with the first standardized CICV simulated scenario. The simulated patient was a 30-yr-old male with a fractured mandible that needed fixation. After performing intravenous induction with propofol, fentanyl, and rocuronium, an actor playing the role of a junior resident called for the participant’s help when the actor/resident was unable to intubate or ventilate after two laryngoscopies. The participant then took over the scenario. The content of the scenario was not previously disclosed. When the participant entered the simulation room, the monitors initially showed an oxygen saturation of 89%, blood pressure of 150/90 mmHg, and sinus tachycardia on ECG at a rate of 110 beats·min−1. Oxygen saturation decreased by 10% every minute, independently of any other maneuver. The saturation would remain at 60-65% if attempts to ventilate the simulated lungs were maintained, and it would decrease to 40-50% (and eventually to “poor signal”) if no ventilation attempt was provided throughout the scenario. The monitoring alarms were set at the highest volume. The mannequin’s airway anatomy was changed so that ventilation could only be re-established if a cricothyroidotomy were performed: the simulated cervical spine was immobilized, the tongue was made edematous, and the vocal cords were adducted. The session was videotaped to be used in subsequent debriefing and collection of data.
After the first CICV scenario, a personalized one-hour video-assisted debriefing with an expert was provided. The debriefing included a review of the guidelines for management of the emergency airway according to the ASA difficult airway algorithm and practical “hands-on” instructions on percutaneous cricothyroidotomy insertion. The same individual conducted all debriefing sessions (L.S.).
Participants underwent a second simulation scenario immediately after the debriefing, unaware that it would be a repetition of the first CICV scenario. The second CICV session was also videotaped for data collection purposes.
Outcome measurements
Our primary outcome measure was the number of participants who showed major deviations from the ASA difficult airway algorithm.4 Specifically: 1) “more than two laryngoscopy attempts”; 2) “use of fibreoptic bronchoscope”; 3) “bypass of laryngeal mask airway attempt”; 4) “failure to call for anesthetic help”; and 5) “failure to call for surgical help”. Secondary outcome measures included: 1) “time to re-establish ventilation”; 2) “time to initiate cricothyroidotomy”; 3) “time to call for anesthetic help”; and 4) “time to call for surgical help”. All simulation sessions were videotaped and randomized for analysis by two expert evaluators who were blinded to the order of the videotapes. The “time to achieve ventilation” was defined from the moment the subject entered the room until ventilation was achieved through the cricothyroidotomy. “Time to initiate cricothyroidotomy” was defined from the moment the subject entered the room until the subject grasped the first instrument on the cricothyroidotomy kit. “Time to call for help”, anesthetic or surgical, was defined from the moment the subject entered the room to the moment help was called.
Sample size and statistical considerations
The number of participants was a convenience sample determined by the number of consultant anesthesiologists in the department of anesthesiology at St. Michael’s Hospital, Toronto. In addition, based on a previous study involving simulators,11 we hypothesized that approximately 50% of the participants would make at least one major deviation from the ASA difficult airway algorithm at baseline (1st CICV). A sample size of 40 would provide 81.6% power to detect a minimum 30% decrease in the proportion of participants committing major deviations from the first to the second session, considering an α error of 0.05.
Analysis was performed using SPSS 11.0 software (Chicago, IL, USA). We analyzed the differences in the primary outcome data between the first and second sessions - number of participants committing a specific deviation - using Fisher’s exact test. The same test was used when comparing the number of participants committing at least one major deviation between sessions. A two-tailed P value of < 0.05 was considered significant.
Secondary outcome measures, including times to call for help, times to start cricothyroidotomy, and times to achieve ventilation - were compared using paired Student’s t tests. A P value < 0.05 was considered significant.
Results
Forty consultant anesthesiologists from our institution were invited to participate. Thirty-eight anesthesiologists agreed to participate; two refused participation for personal reasons, and two participants were excluded from the study due to technical problems. The demographic characteristics of our participants are shown in Table 1.
There were no differences between the first and second sessions regarding the number of participants committing major deviations, such as, “more than two laryngoscopy attempts” (0 vs 2 pre-post; P = 0.493), “use of fibreoptic bronchoscope (8 vs 7 pre-post; P = 1.000), “bypass of laryngeal mask airway attempt” (7 vs 13 pre-post; P = 0.187), and “failure to call for anesthetic help” (12 vs 8, pre-post; P = 0.430). However, more participants showed a “failure to call for surgical help” in the second session (7 vs 16 pre-post; P = 0.042). Means times to initiate cricothyroidotomy and times to achieve ventilation were significantly better in the second session (205.5 ± 61.3 sec vs 179.7 ± 65.1 sec; P = 0.011 and 356.9 ± 117.2 sec vs 269.4 ± 77.43 sec; P = 0.0002, respectively). These and other results are summarized in Tables 2 and 3.
Discussion
Although there is compelling data showing that simulation training is an effective method to improve anesthesia technical psychomotor skills14-16 and to disseminate algorithms and guidelines for anesthesia residents,12,17 there is a paucity of studies that evaluate the effectiveness of simulation pertaining to implementation of patient care algorithms in the anesthesia literature.
The observation of the first session in this study outlines a CICV scenario being managed by attending anesthesiologists at a tertiary care institution. Despite worldwide dissemination of similar emergency airway practice guidelines2-4 and proper accreditation in continuing medical education of our faculty, almost two-thirds of these anesthesiologists had at least one major deviation from the ASA difficult airway guidelines. Despite our focus on these deviations during an intense personalized one-hour video-assisted debriefing with an expert, it is clear from our data that no important changes in decision-making were observed during a second CICV scenario, where, surprisingly, 75% of the participants committed at least one major deviation despite being individually educated and debriefed.
Many factors come into consideration18 when interpreting these results, although lack of familiarity with the ASA difficult airway guidelines is not one of them. We are aware that adult learners are often resistant to changing their clinical practice patterns for a number of different reasons.19,20 Firstly, previous experiences play a great role on decision-making processes. The consultant anesthesiologists in this study demonstrated a preference to manage the CICV scenario based on their current skills and knowledge.21 Unlike residents in training, perhaps consultant anesthesiologists predetermined their own modifications for difficult airway algorithms based on their own level of comfort with airway adjuncts and their interpretation of the airway literature.
Secondly, we recognize that learning a task must have relevance to one’s daily practice. Adults have to experience a need to learn in order to cope with real-life tasks or problems.21 Fortunately, a CICV situation is quite rare, and few anesthesiologists will ever have performed a cricothyroidotomy during their careers.18,22 Most participants in this study had not previously encountered such a situation in real-life (Table 1).
Thirdly, adult learners need to be willingly involved with learning.21 Our informed consent invited participants to participate in two unspecific “simulation sessions”. The participants were unaware that they were going to manage two stressful CICV scenarios back-to-back and have a debriefing session with an expert. According to Knowles et al.,23 “adults resent and resist situations in which they feel others are imposing their wills on them.” For instance, this argument might explain possible differences if a comparison were made between our learning results and the learning results from an airway workshop where participants searched willingly for that specific knowledge.
In the second session, after the anesthesiologists became more familiarized with the performance of cricothyroidotomy, they had a tendency to bypass the request for surgical help. Perhaps the study participants deemed surgical help no longer necessary when they became comfortable in performing a cricothyroidotomy. This behaviour is in accordance with the heuristic approach to problem solving24 rather than the algorithmic approach. The heuristic approach, wherein one’s process of decision-making relies on experience, intuition, and skill to reach the final goal, may be used to decrease cognitive strain and to simplify information processing - ventilation, in our case - without exploring other suitable alternatives. The same idea also explains the observation that participants took significantly less time in the second session to start the cricothyroidotomy and to achieve ventilation (Table 3). Expert clinicians make use of heuristic approaches on a regular basis;24 however, this approach carries higher risks of systematic errors in judgement.25-27
Professionals knowingly tend to stop searching for new knowledge once they feel comfortable in their jobs.27-29 Due to the rarity of CICV situations,30 experienced anesthesiologists may tend to assume that specific training on airway tools is unnecessary.1,31 This behaviour, i.e., being excessively reliant on expertise, may well be extrapolated to other anesthesiologists.1,22,31
As previously discussed, adults learn more when they willingly search for knowledge.21 However, since consultant physicians tend to participate in meeting workshops where they are already knowledgeable about the topics being addressed32 and since they often fail in their self-assessment of educational needs,32,33 more effective approaches are needed to engage consultant anesthesiologists in maintaining excellence in the management of the emergency airway.28
The role of simulation and its effective use in training experienced physicians to improve their adherence to emergency airway guidelines are still to be determined. The repetition of simulation sessions may be useful for improving adherence to algorithms in general.11 However, given the costs of simulation teaching and displacement of experienced physicians from their workplace, there is a definite need for a more reliable and effective way to approach these expert participants.
Our data show that only 58% of our participants were previously exposed to a simulation session. The majority (84.4%) had no airway training simulation before our study (Table 1). In addition, due to the rarity of CICV situations, most participants may not have had clinical experience going through the entire ASA difficult airway algorithm. Despite their lack of improvement in adherence following the debriefing, our data suggest that our simulation sessions may have helped participants to reduce the delay in recognizing the impossibility of tracheal intubation and the necessity for infraglottic intervention.
Our study has several limitations. Our “airway cart” did not offer all the airway adjuncts listed in the ASA difficult airway algorithm. As options in managing a CICV situation, such as the “esophageal-tracheal combitube” and the “jet-ventilator” were not available, participants may have been obliged to readapt to a “new algorithm” from the one they already had on their minds. This detail may have altered their adherence to the ASA difficult airway algorithm. However, we chose not to include these airway items as their inclusion may have been outdated and controversial.34,35
Finally, experienced learners may need time to reflect on their learning intervention and their experience. Given that the second scenario was introduced immediately after the debriefing, it is possible that there was insufficient time for the participants to process the information and readapt their practice to their new knowledge.
In conclusion, this study shows that consultant anesthesiologists do not routinely adhere to the ASA difficult airway guidelines. Lack of awareness of these guidelines does not appear to have been a factor. A more likely explanation for variances in airway management is that consultant anesthesiologists adapt the ASA algorithm based on their own clinical experience, airway equipment skill, and interpretation of the airway literature. Further research should be undertaken to explore other educational approaches that may be more effective in increasing consultant anesthesiologists’ adherence to published difficult airway guidelines.
References
Crosby E. The unanticipated difficult airway–evolving strategies for successful salvage. Can J Anesth 2005; 52: 562-7.
Crosby ET, Cooper RM, Douglas MJ, et al. The unanticipated difficult airway with recommendations for management. Can J Anaesth 1998; 45: 757-76.
Henderson JJ, Popat MT, Latto IP, Pearce AC; Difficult Airway Society. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia 2004; 59: 675-94.
American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2003; 98: 1269-77
Li G, Warner M, Lang BH, Huang L, Sun LS. Epidemiology of anesthesia-related mortality in the United States, 1999–2005. Anesthesiology 2009; 110: 759-65.
The Canadian Medical Protective Association. Anesthesia Airway Management - An Analysis of the CMPA’s Closed Legal Actions 1993-2003. CMPA; 2008: 1-4. Available from URL: http://www.cmpa-acpm.ca/cmpapd04/docs/resource_files/risk_id/2005/pdf/com_ri0507-e.pdf (accessed March 2010)
Cheney FW, Posner KL, Lee LA, Caplan RA, Domino KB. Trends in anesthesia-related death and brain damage: a closed claims analysis. Anesthesiology 2006; 105: 1081-6.
Peterson GN, Domino KB, Caplan RA, Posner KL, Lee LA, Cheney FW. Management of the difficult airway: a closed claims analysis. Anesthesiology 2005; 103: 33-9.
Berkow LC, Greenberg RS, Kan KH, et al. Need for emergency surgical airway reduced by a comprehensive difficult airway program. Anesth Analg 2009; 109: 1860-9.
Riley RH, Strang T, Rao S. Survey of airway skills of surgeons in Western Australia. Anaesth Intensive Care 2009; 37: 630-3.
Chopra V, Gesink BJ, de Jong J, Bovill JG, Spierdijk J, Brand R. Does training on an anaesthesia simulator lead to improvement in performance? Br J Anaesth 1994; 73: 293-7.
Kuduvalli PM, Jervis A, Tighe SQ, Robin NM. Unanticipated difficult airway management in anaesthetised patients: a prospective study of the effect of mannequin training on management strategies and skill retention. Anaesthesia 2008; 63: 364-9.
John B, Suri I, Hillermann C, Mendonca C. Comparison of cricothyroidotomy on manikin vs. simulator: a randomised cross-over study. Anaesthesia 2007; 62: 1029-32.
Chandra DB, Savoldelli GL, Joo HS, Weiss ID, Naik VN. Fiberoptic oral intubation: the effect of model fidelity on training for transfer to patient care. Anesthesiology 2008; 109: 1007-13.
Friedman Z, You-Ten KE, Bould MD, Naik V. Teaching lifesaving procedures: the impact of model fidelity on acquisition and transfer of cricothyrotomy skills to performance on cadavers. Anesth Analg 2008; 107: 1663-9.
Wong DT, Prabhu AJ, Coloma M, Imasogie N, Chung FF. What is the minimum training required for successful cricothyroidotomy?: a study in mannequins. Anesthesiology 2003; 98: 349-53.
Schaefer JJ 3rd. Simulators and difficult airway management skills. Paediatr Anaesth 2004; 14: 28-37.
Wong DT, Lai K, Chung FF, Ho RY. Cannot intubate-cannot ventilate and difficult intubation strategies: results of a Canadian national survey. Anesth Analg 2005; 100: 1439-46.
Scott SM, Spencer B, Thomas AM. Learning for life: Canadian Readings in Adult Education. Toronto: Thompson Educational Publishing Inc; 1998.
Boufettal H, Hermas S, Noun M, Samouh N. Medical andragogy (French). J Gynecol Obstet Biol Reprod (Paris) 2009; 38: 445-7.
Knowles MS. Application in continuing education for the health professions: chapter five of “Andragogy in Action”. Mobius 1985; 5: 80-100.
Rosenblatt WH, Wagner PJ, Ovassapian A, Kain ZN. Practice patterns in managing the difficult airway by anesthesiologists in the United States. Anesth Analg 1998; 87: 153-7.
Knowles MS, Holton E, Swanson RA. The Adult Learner: The Definitive Classic in Adult Education and Human Resource Development. 5th ed. Houston, TX: Gulf Publishing Co.; 1998.
Kremer MJ, Faut-Callahan M, Hicks FD. A study of clinical decision making by certified registered nurse anesthetists. AANA J 2002; 70: 391-7.
Kahneman D, Tversky A. On the study of statistical intuitions. Cognition 1982; 11: 123-41.
Kahneman D, Tversky A. Variants of uncertainty. Cognition 1982; 11: 143-57.
Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med 2005; 142: 260-73.
Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med 2004; 79(10 Suppl): S70-81.
Weinger MB. Experience not equal expertise: can simulation be used to tell the difference? Anesthesiology 2007; 107: 691-4.
Benumof J. Airway Management: Principles and Practice. St. Louis: Mosby; 1996.
Kristensen MS, Moller J. Airway management behaviour, experience and knowledge among Danish anaesthesiologists–room for improvement. Acta Anaesthesiol Scand 2001; 45: 1181-5.
Fletcher P. Continuing medical education in a district general hospital: a snapshot. Med Educ 2001; 35: 967-72.
Jankowski J, Crombie I, Block R, Mayet J, McLay J, Struthers AD. Self-assessment of medical knowledge: do physicians overestimate or underestimate? J R Coll Physicians Lond 1991; 25: 306-8.
Vezina MC, Trepanier CA, Nicole PC, Lessard MR. Complications associated with the Esophageal-Tracheal Combitube in the pre-hospital setting. Can J Anesth 2007; 54: 124-8.
Cook TM, Alexander R. Major complications during anaesthesia for elective laryngeal surgery in the UK: a national survey of the use of high-pressure source ventilation. Br J Anaesth 2008; 101: 266-72.
Funding sources
This study used departmental funds and resources.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is accompanied by an editorial. Please see Can J Anesth 2010; 57(7).
Rights and permissions
About this article
Cite this article
Borges, B.C.R., Boet, S., Siu, L.W. et al. Incomplete adherence to the ASA difficult airway algorithm is unchanged after a high-fidelity simulation session. Can J Anesth/J Can Anesth 57, 644–649 (2010). https://doi.org/10.1007/s12630-010-9322-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-010-9322-4