Abstract
Purpose
Pregabalin exhibits potent anticonvulsant, analgesic, and anxiolytic activity in animal models. However, few studies have evaluated pregabalin’s potential peripheral effects on neuropathic pain. The aim of this study was to evaluate the peripheral analgesic effects of pregabalin in a rat model of neuropathic pain.
Methods
Male Sprague-Dawley rats were prepared by ligating the left L5 and L6 spinal nerves to produce neuropathic pain. Sixty rats with neuropathic pain were randomly assigned to six groups. Normal saline (control) and pregabalin (10, 20, 30, and 50 mg·kg−1) were administered to the plantar surface of the affected left hind paw. Pregabalin (50 mg·kg−1) was administered into the unaffected contralateral paw in order to determine its systemic effect. Responses to mechanical, cold, and heat stimulation were recorded at 15, 30, 60, 90, 120, 150, and 180 min after drug administration. Rotarod performance was measured to detect drug-induced side effects, including sedation and reduced motor coordination.
Results
Saline injected into the affected paw and a pregabalin dose of 50 mg·kg−1 injected into the contralateral paw showed no differences for mechanical, cold, and heat allodynia. Administration of pregabalin to the affected left hind paw in the dose range of 10-50 mg·kg−1 resulted in a dose-dependent increase in thresholds to mechanical, cold, and heat stimulation.
Conclusion
Peripherally administered pregabalin attenuates mechanical, cold, and heat allodynia in a rat model of neuropathic pain.
Résumé
Objectif
La prégabaline démontre une puissante activité anticonvulsivante, analgésique et anxiolytique dans les modèles animaux. Néanmoins, il n’existe que peu d’études ayant évalué les effets périphériques potentiels de la prégabaline sur la douleur neuropathique. L’objectif de cette étude était d’examiner les effets analgésiques périphériques de la prégabaline dans un modèle de douleur neuropathique chez le rat.
Méthode
Des rats Sprague-Dawley mâles ont été préparés en ligaturant les nerfs rachidiens L5 et L6 afin de créer une douleur neuropathique. Soixante rats souffrant de douleur neuropathique ont été aléatoirement répartis en six groupes. Du sérum physiologique (témoin) et de la prégabaline (10, 20, 30, et 50 mg·kg−1) ont été administrés à la surface plantaire de la patte arrière gauche affectée. De la prégabaline (50 mg·kg−1) a été administrée à la patte controlatérale non affectée afin de déterminer son effet systémique. Les réactions aux stimulations mécaniques, au froid et à la chaleur ont été enregistrées à 15, 30, 60, 90, 120, 150 et 180 min après l’administration du médicament. La performance au test de la tige tournante a été mesurée afin de dépister les effets secondaires provoqués par le médicament, notamment la sédation et une réduction de la coordination motrice.
Résultats
Le sérum physiologique injecté dans la patte affectée et une dose de 50 mg·kg−1 de prégabaline injectée dans la patte controlatérale ont démontré des réactions semblables concernant l’allodynie mécanique, au froid et à la chaleur. L’administration de prégabaline à la patte arrière gauche affectée, dans une marge posologique de 10 à 50 mg·kg−1, a causé une augmentation dose-dépendante des seuils de stimulation mécanique, au froid et à la chaleur.
Conclusion
La prégabaline, lorsqu’elle est administrée aux nerfs périphériques, atténue l’allodynie mécanique, au froid et à la chaleur dans un modèle de douleur neuropathique chez le rat.
Similar content being viewed by others
Neuropathic pain remains an important clinical problem for which the mechanism has not been clearly determined.1 Conventional analgesics, such as non-steroidal anti-inflammatory drugs and opioids, have limited efficacy in the treatment of neuropathic pain. Also, they are associated with mild and occasionally serious side effects.2 Presently, anticonvulsants (e.g., gabapentin and carbamazepine), antidepressants, and N-methyl-D-aspartate (NMDA) antagonists are prescribed, but their efficacy for the treatment of neuropathic pain is limited at best.1 , 3
Pregabalin, known formerly as S(+)-3-isobutyl-gamma aminobutyric acid (isobutylgaba) or CI-1008, is a potent ligand for the alpha-2-delta (α2δ) subunit of the voltage-gated calcium channels in the central nervous system. Pregabalin exhibits potent anticonvulsant, analgesic, and anxiolytic activity in a range of animal models.4 Pregabalin has been shown to relieve the pain induced by formalin,5 kaolin, and carrageenan.6 Systemic or intrathecal administration of pregabalin has been shown to attenuate the nociceptive signals in a rat model of neuropathic pain.7-10
In the clinical setting, systemic pregabalin has been shown to reduce postoperative pain.11 , 12 Recent clinical reports suggest that systemic pregabalin is effective for treating neuropathic pain associated with postherpetic neuralgia and diabetic peripheral neuropathy.13-15 Although pregabalin has been shown to relieve neuropathic pain, both in preclinical models and clinically, the peripheral analgesic effect of this drug in animal neuropathic pain models has not been profiled for mechanical, cold, and heat hypersensitivity. In addition, while the antinociceptive effect of pregabalin may be produced by spinal, supraspinal, or peripheral inhibitory action on ectopic afferent discharge, the primary site of the drug’s anti-allodynic action remains the subject of debate.8 , 16 , 17
Therefore, to determine if pregabalin has a peripheral site of action, we examined the anti-allodynic effects of intraplantar injection of pregabalin by observing the withdrawal responses to mechanical, cold, and heat stimuli in a rat model of neuropathic pain.
Methods
Animals
All experimental procedures were approved by our institutional animal investigation committee. In this study, we used male Sprague-Dawley rats each weighing 150-180 g, and we provided them with food and water ad libitum. The rats were housed in groups of three to four in plastic cages with soft bedding, and they were maintained on a 12:12 hr light-dark cycle. Before conducting this experiment, the experimental rats were allowed to adjust to their environment for at least seven days.
Spinal nerve ligation
We used the method of Kim and Chung18 to produce the neuropathic pain model by ligating the left L5 and L6 spinal nerves. After the surgery, the rats were allowed to recover for seven days before we started the behavioural testing. The animals we used in the tests were those that showed a foot withdrawal response to von Frey filaments (Semmes Weinstein von Frey esthesiometer, Stoelting Co., IL, USA), with an applied bending force of ≤ 4 g considered neuropathic.19 The rats that exhibited motor deficiency, such as paw dragging or limping, or those that failed to exhibit subsequent mechanical allodynia were excluded from any further testing.
Drug administration
Pregabalin (donated from Pfizer Inc, Groton, CT, USA) was reconstituted in a 0.9% saline solution. All of the pregabalin or saline doses were administered in one hind paw as a 50 μL bolus. The rats with neuropathic pain were randomized to one of six groups. The groups consisted of: 1) saline only – NS; 2) pregabalin 10 mg·kg−1 – PRGB10; 3) pregabalin 20 mg·kg−1 – PRGB20; 4) pregabalin 30 mg·kg−1 – PRGB30; 5) pregabalin 50 mg·kg−1 - PRGB50; and 6) pregabalin 50 mg·kg−1 injected into the right paw – PRGB 50Rt. We included PRGB 50Rt to control for the systemic effect of subcutaneously injected pregabalin. The needle puncture to deliver the study drug solution was made via the plantar skin surface. The solution was then placed in the subcutaneous space just proximal to the third metatarsal bone.20
Behavioural tests
All behavioural tests were conducted at fixed times (1 p.m. to 6 p.m.) to avoid bias attributable to the influence of circadian rhythm, and the tests were performed by the same person who was blinded to the study solution. Mechanical, cold, and heat allodynia were assessed before intraplantar injection and also at 15, 30, 60, 90, 120, 150, and 180 min after the injection. After intraplantar injection, the rats were placed on a metal mesh covered with a plastic dome (8 × 8 × 18 cm) for the assessment of mechanical and cold allodynia. The dosage regimen and observation times were based on the preliminary experimental results and our previous experience.
The thresholds for mechanical allodynia were measured with a series of von Frey filaments (0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0, and 26.0 g). The third metatarsal bone area of the left hind paw was stimulated with von Frey filaments at three- to four-second intervals using the up-down method.21 We recorded the minimal pressure level (in grams) that initiated a response. If the strongest filament did not elicit a response, then the threshold was recorded as 26.0 g.
Cold allodynia was measured as the number of foot withdrawal responses (lifting, shaking, or licking) after the application of cold stimuli to the plantar surface of the paw.22 , 23 A drop of 100% acetone was gently applied to the left heel of the rat with a syringe connected to a thin polyethylene tube. The test was repeated five times with an interval of approximately three to five minutes between each test. The response frequency to acetone was expressed as a percent response frequency, i.e., the number of paw withdrawals/the number of trials × 100. The noxious heat thresholds for heat allodynia were measured using an increasing-temperature hot plate (IITC Life Science, Woodland Hills, CA, USA) that was recently validated.24 After habituation, the rats were placed onto the plate that was then heated from a starting temperature of 30°C at a rate of 12°C·min−1 until the animal showed nocifensive behaviour involving either hindpaw. The typical response was hindpaw licking, shaking, and lifting. Jumping was rare but observed. The corresponding plate temperature was considered the noxious heat threshold.25 Changes of the locomotor function of the neuropathic rats were evaluated by rotarod testing (Acceler rota-rod for rats 7750; Ugo Basile, Comerio-Varese, Italy). The rats with neuropathic pain were acclimatized to the revolving drum, and they were desensitized to manual handling to ameliorate any stress during testing. The rats were given three training trials on the revolving drums (10-15 rpm) for two days, and those able to remain on the revolving drum for a minimum of 150 sec were selected for drug testing. The mean of three training runs served as a control performance time. The rotarod performance time was measured at 15, 30, 60, 90, 120, 150, and 180 min after intraplantar injection. Each test was performed three times at five-minute intervals, and the mean values were compared. The tactile, cold, and heat tests were performed simultaneously, i.e., each test was repeated in order at each time interval, and, due to the time required to perform the test, only the rotarod testing was performed separately at each recording interval.
Statistical analysis
The results are expressed as the mean ± standard error of mean (SEM). Statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Repeated measures analysis of variance (RMANOVA) was used to test for group-time interaction. When RMANOVA was significant, post hoc testing was done using Dunnett’s test. A P value of < 0.01 was considered significant. For dose-response curves, withdrawal data were converted to a percentage of the maximum possible effect (%MPE) using the following formulas: 1) The %MPE for mechanical allodynia = (post drug threshold-baseline threshold) / (cut-off threshold-baseline threshold) × 100; and 2) The %MPE for cold and heat allodynia = (baseline withdrawal frequency-post drug withdrawal frequency)/(baseline withdrawal frequency) × 100. Dose-response data were analyzed using one-way ANOVA.
Results
Neuropathic pain behaviour developed in 81% of the experimental rats within five days after performing spinal nerve ligation. These animals appeared to be healthy without any evidence of paralysis or limping. Throughout the two-week experimental period, the degree of allodynia experienced by the rats was consistently maintained.
Evaluation of the systemic reaction to pregabalin
When compared with the control group, systemically absorbed pregabalin at a dose of 50 mg·kg−1 injected into the right paw resulted in no differences in 1) the withdrawal thresholds in response to mechanical stimulation (Figure 1); 2) the withdrawal frequencies in response to cold stimulation (Figure 3); and 3) the noxious heat thresholds to heat stimulation of the injured left paw (Figure 5).
In the rat model of neuropathic pain, the time course of the paw withdrawal threshold to mechanical stimuli applied to the plantar surface of the affected left paw with von Frey filaments. *P < 0.001 vs the saline group by repeated measures analysis of variance (ANOVA) and Dunnett’s test. †P < 0.001 vs the pre-administration time by repeated measures ANOVA and Dunnett’s test
Mechanical allodynia
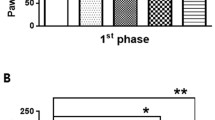
All of the rats developed mechanical allodynia after spinal nerve ligation with a foot withdrawal threshold below 4 g. Before normal saline administration, the withdrawal threshold in the control group was 1.1 ± 0.1 g, and after normal saline administration, the withdrawal thresholds were 1.3 ± 0.1, 1.4 ± 0.1, 1.7 ± 0.3, 1.5 ± 0.1, 1.2 ± 0.1, 1.1 ± 0.1, and 1.1 ± 0.1 g at 15, 30, 60, 90, 120, 150, and 180 min, respectively. At 60 min after administering pregabalin 10 mg·kg−1, the withdrawal threshold increased compared with pre-administration; however, there was no difference in the withdrawal threshold compared with the control group (P < 0.001). The withdrawal threshold time increased to 60 min for 20 mg·kg−1, from 15 to 120 min for 30 mg·kg−1, and from 15 to 150 min for 50 mg·kg−1, compared with the control group (P < 0.001) (Figure 1). Peripheral administration of pregabalin dose-dependently attenuated mechanical allodynia (P < 0.001) (Figure 2).
In the pregabalin groups, dose-response curves from the peak effects of percent maximal possible effect (%MPE) for mechanical anti-allodynia. These curves show a dose-dependent mechanical anti-allodynic effect. Each line represents the mean ± standard error of mean (SEM) for ten rats. Doses (mg·kg−1) are represented logarithmically on the x axis and peak %MPE is represented on the y axis. †P < 0.001 vs 10 mg·kg−1, *P < 0.001 vs 20 mg·kg−1, ∫∫P < 0.001 vs 30 mg·kg−1, as determined by one-way ANOVA
Cold allodynia
Before drug administration, all of the groups showed withdrawal frequencies of ≥ 92% when 100% acetone was applied, and there were no differences between the groups. After administering pregabalin 10 mg·kg−1 and 20 mg·kg−1, the withdrawal frequency decreased from 30 to 60 min and from 30 to 90 min, respectively, compared with pre-administration (P < 0.001); whereas there was no difference compared with the control group. Compared with the control group, administration of pregabalin reduced the withdrawal frequency to acetone application from 15 to 30 min for 30 mg·kg−1 and from 15 to 150 min for 50 mg·kg−1 (P < 0.001) (Figure 3). Peripheral administration of pregabalin dose-dependently attenuated cold allodynia (P < 0.019) (Figure 4).
In the rat model of neuropathic pain, time course of the paw withdrawal frequency to cold stimuli using 100% acetone. *P < 0.001 vs the saline group by repeated measures analysis of variance (ANOVA) and Dunnett’s test. †P < 0.001 vs the pre-administration time by repeated measures ANOVA and Dunnett’s test
Dose-response curves from the peak effects of percent maximal possible effect (%MPE) for cold anti-allodynia in the pregabalin groups. These curves show a dose-dependent cold anti-allodynic effect. Each line represents the mean ± standard error of mean (SEM) for ten rats. Doses (mg·kg−1) are represented logarithmically on the x axis and peak %MPE is represented on the y axis. †P < 0.019 vs 10 mg·kg−1, *P < 0.019 vs 20 mg·kg−1, ∫∫ < 0.019 vs 30 mg·kg−1, as determined by one-way analysis of variance
Heat allodynia
When the rats with neuropathic pain were placed onto the increasing-temperature hot plate, all of the groups showed heat thresholds of 45.3 ± 0.1°C before drug administration. Administration of pregabalin increased the noxious heat threshold time to heat allodynia from 15 to 30 min for 10 mg·kg−1, from 15 to 60 min for 20 mg·kg−1, from 15 to 90 min for 30 mg·kg−1, and from 15 to 120 min for 50 mg·kg−1 (P < 0.001) (Figure 5). Peripheral administration of pregabalin dose-dependently attenuated heat allodynia (P < 0.001) (Figure 6).
In the rat model of neuropathic pain, time course of the paw withdrawal threshold to heat stimuli using an increasing-temperature hot plate. *P < 0.001 vs the saline group by repeated measures analysis of variance (ANOVA) and Dunnett’s test. †P < 0.001 vs the pre-administration time by repeated measures ANOVA and Dunnett’s test
Dose-response curves from the peak effects of percent maximal possible effect (%MPE) for heat anti-allodynia in the pregabalin groups. These curves show a dose-dependent heat anti-allodynic effect. Each line represents the mean ± standard error of mean (SEM) for ten rats. Doses (mg·kg−1) are represented logarithmically on the x axis and peak %MPE is represented on the y axis. †P < 0.001 vs 10 mg·kg−1, *P < 0.001 vs 20 mg·kg−1, ∫∫ < 0.001 vs 30 mg·kg−1, as determined by one-way ANOVA
Rotarod performance
There was no significant change between the rats treated with saline and the rats treated with pregabalin 10-30 mg·kg−1 with regard to their competence to perform the rotarod test. In contrast, when treated with pregabalin 50 mg·kg−1, the rats’ rotarod performance time at 15, 30, 60, 90, 120, 150, and 180 min decreased from the statistical cut-off time (150 sec) to 127.8 ± 4.9, 114.4 ± 4.0, 127.7 ± 3.5, 133.7 ± 3.4, 140.9 ± 3.9, 145.4 ± 3.1, and 148.5 ± 1.5 sec, respectively. The rats’ rotarod performance time decreased during the 15 to 90 min period after pregabalin 50 mg·kg−1 was administered. At 120, 150 and 180 min, the rats’ rotarod performance time gradually recovered and did not differ significantly from the statistical cut-off time (150.0 sec) (P < 0.001) (Figure 7).
Discussion
This study demonstrates that intraplantar administered pregabalin is associated with a dose–dependent anti-allodynic effect in a rat model of neuropathic pain. Local injection of pregabalin resulted in a dose-dependent increase in threshold to mechanical, cold, and heat stimulation. However, compared with the control group, injection of pregabalin 50 mg·kg−1 to the unaffected right paw did not elicit an anti-allodynic effect at any point during the study. This showed that there was no anti-allodynic action on the left paw as a result of any systemic effect at these administered doses. These results highlight the peripheral action of pregabalin in a rat model of neuropathic pain.
It is not clear how pregabalin exerts an anti-allodynic effect on neuropathic pain. In previous studies, it was either stated or assumed that the antihyperalgesic effects of pregabalin were achieved through a supraspinal or spinal site of action.10 , 26 Han et al.10 reported that intraperitoneal and intrathecal pregabalin in a rat model of neuropathic pain decreased the tactile and cold allodynia in a dose-dependent manner. Autoradiographic analysis of R217A mutant mice has shown that the mutation to α2δ-1 substantially reduces specific pregabalin binding in the central nervous system regions that are known to preferentially express the α2δ-1 protein, notably the neocortex, hippocampus, basolateral amygdala, and spinal cord.27 This finding provides evidence that the α2δ-1 subunit of the voltage-gated calcium channels is the major binding protein for pregabalin in the CNS.27
Pregabalin may also exert its mechanism of action peripherally. Carlton and Zhou5 reported that local peripheral injection of S-(+)-3-isobutylgaba reduced the formalin-induced nociceptive behaviours. Chen et al.17 reported that intravenous injection of pregabalin dose-dependently attenuated the tactile allodynia and thermal hyperalgesia in sciatic nerve-ligated neuropathic rats. Furthermore, pregabalin significantly inhibited the ectopic discharges from injured afferent neurons in a dose-dependent manner. The preceding data strongly suggest that the analgesic effect of pregabalin on neuropathic pain is mediated by its peripheral inhibitory action on the generation of ectopic discharges caused by nerve injury. The effect of pregabalin on ectopic afferent activity may contribute to its analgesic action by directly eliminating the nociceptive afferent input to the spinal cord. Attenuation of the nociceptive input into the spinal cord would reduce the level of central sensitization of the dorsal horn cells.
Pregabalin is also known to suppress the release of substance P and calcitonin gene-related peptide (CGRP).28 Peripherally-released substance P and CGRP were noted to mediate mechanical hyperalgesia in a peripheral neuropathic pain model.29 Therefore, pregabalin administered peripherally may affect the release of these mediators and alleviate the hyperalgesia in this type of pain model.
Immunohistochemical staining for the glutamate receptor subtypes NMDA, kainate, and AMPA has been shown to produce labelled unmyelinated axons in the glabrous skin of the rat hindpaw.30 Injecting glutamate into the rat hindpaw has produced behavioural changes that can be interpreted as mechanical allodynia and mechanical hyperalgesia. It has also been shown that peripheral glutamate receptors have been involved in mechanical hyperalgesia in a peripheral neuropathic pain model.31 It has been suggested that pregabalin may reduce the release of glutamate.32 Therefore, blockade of the peripheral glutamate receptors by pregabalin in our current neuropathic pain model experiment may be another mechanism of action for reducing the allodynia.
Only three studies have described the peripheral effect of pregabalin in a rat model. Carlton and Zhou5 reported that local peripheral injection of pregabalin reduced formalin-induced nociceptive behaviours, but they used a formalin rat model and they assessed the flinching and lifting/licking behaviours. In contrast to Carlton’s experiment,5 we used the spinal nerve ligation model and we evaluated the mechanical, cold, and heat allodynia. Chen et al.17 reported that intravenous injection of pregabalin dose-dependently attenuated tactile allodynia and thermal hyperalgesia in sciatic nerve-ligated neuropathic rats, but they did not assess cold allodynia. We administered pregabalin peripherally, not intravenously, and we evaluated responses to mechanical, cold, and heat allodynia following spinal nerve ligation. Field et al.11 reported that subcutaneous injection of gabapentin and pregabalin dose-dependently block the development or maintenance of hyperalgesia and allodynia in a rat model of postoperative pain. Yet, administration of gabapentin into the hind paw failed to block both the static and dynamic allodynia induced by streptozocin in rats.33 This discrepancy with our study is possibly due to the different animal models (streptozocin vs spinal nerve ligation) and the different types and doses of drugs (gabapentin 1-100 μg/animal vs pregabalin 10-50 mg·kg−1).
Pregabalin-related side effects include mild-to-moderate central nervous system disturbances, such as dizziness, somnolence and ataxia, dose-dependent peripheral edema, weight gain,34 , 35 myoclonus,36 , 37 a cortical negative form of myoclonus,38 and exacerbation of heart failure.39 , 40 These side effects are clinically important because they restrict the use of pregabalin for continuous treatment of patients with chronic pain. In addition, if the neuropathic rats in our study were sedated by pregabalin, then the effect on mechanical, cold, and heat allodynia would have been exaggerated. Therefore, we performed the rotarod test to evaluate the effect of pregabalin on motor coordination to exclude the sedation-induced anti-allodynic effect. We investigated such side effects as sedation or motor disturbance by administering different concentrations of pregabalin. Only the rats with the highest dose (50 mg·kg−1) showed a reduction of rotarod performance during the first 15 to 120 min. Since the rotarod performance was decreased only at the highest dose, it is unlikely that the anti-allodynic effect of pregabalin at 10-30 mg·kg−1 is due to the sedative effect of pregabalin.
In conclusion, intraplantar administered pregabalin in the dose range of 10-50 mg·kg−1 attenuated the mechanical, cold, and heat allodynia exhibited by spinal nerve ligated rats. Sedation and motor disturbance were only observed at the highest dose (50 mg·kg−1), suggesting that sedation was not the mechanism for attenuation of allodynia. These results suggest that pregabalin may exert a peripheral analgesic effect in a model of neuropathic pain in a dose range that is not associated with either sedation or motor disturbance. Randomized controlled trials are warranted to confirm these findings in the clinical setting.
References
Colombo B, Annovazzi PO, Comi G. Medications for neuropathic pain: current trends. Neurol Sci 2006; 27(Suppl 2): S183-9.
MacFarlane BV, Wright A, O’Callaghan J, Benson HA. Chronic neuropathic pain and its control by drugs. Pharmacol Ther 1997; 75: 1-19.
McCleane G. Pharmacological management of neuropathic pain. CNS Drugs 2003; 17: 1031-43.
Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia 2004; 45(Suppl 6): 13-8.
Carlton SM, Zhou S. Attenuation of formalin-induced nociceptive behaviors following local peripheral injection of gabapentin. Pain 1998; 76: 201-7.
Houghton AK, Lu Y, Westlund KN. S-(+)-3-isobutylgaba and its steroisomer reduces the amount of inflammation and hyperalgesia in an acute arthritis model in the rat. J Pharmacol Exp Ther 1998; 285: 533-8.
Field MJ, Bramwell S, Hughes J, Singh L. Detection of static and dynamic components of mechanical allodynia in rat models of neuropathic pain: are they signalled by distinct primary sensory neurones? Pain 1999; 83: 303-11.
Wallin J, Cui JG, Yakhnitsa V, Schechtmann G, Meyerson BA, Linderoth B. Gabapentin and pregabalin suppress tactile allodynia and potentiate spinal cord stimulation in a model of neuropathy. Eur J Pain 2002; 6: 261-72.
Takeuchi Y, Takasu K, Ono H, Tanabe M. Pregabalin, S-(+)-3-isobutylgaba, activates the descending noradrenergic system to alleviate neuropathic pain in the mouse partial sciatic nerve ligation model. Neuropharmacology 2007; 53: 842-53.
Han DW, Kweon TD, Lee JS, Lee YW. Antiallodynic effect of pregabalin in rat models of sympathetically maintained and sympathetic independent neuropathic pain. Yonsei Med J 2007; 48: 41-7.
Field MJ, Holloman EF, McCleary S, Hughes J, Singh L. Evaluation of gabapentin and S-(+)-3-isobutylgaba in a rat model of postoperative pain. J Pharmacol Exp Ther 1997; 282: 1242-6.
Hill CM, Balkenohl M, Thomas DW, Walker R, Mathe H, Murray G. Pregabalin in patients with postoperative dental pain. Eur J Pain 2001; 5: 119-24.
Dworkin RH, Corbin AE, Young JP Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2003; 60: 1274-83.
Sabatowski R, Galvez R, Cherry DA, et al; 1008-045 Study Group. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain 2004; 109: 26-35.
Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 2004; 110: 628-38.
Taylor CP, Vartanian MG, Yuen PW, Bigge C, Suman-Chauhan N, Hill DR. Potent and stereospecific anticonvulsant activity of 3-isobutyl GABA relates to in vitro binding at a novel site labeled by tritiated gabapentin. Epilepsy Res 1993; 14: 11-5.
Chen SR, Xu Z, Pan HL. Stereospecific effect of pregabalin on ectopic afferent discharges and neuropathic pain induced by sciatic nerve ligation in rats. Anesthesiology 2001; 95: 1473-9.
Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992; 50: 355-63.
Jang Y, Kim ES, Park SS, Lee J, Moon DE. The suppressive effects of oxcarbazepine on mechanical and cold allodynia in a rat model of neuropathic pain. Anesth Analg 2005; 101: 800-6.
Xie J, Yoon YW, Yom SS, Chung JM. Norepinephrine rekindles mechanical allodynia in sympathectomized neuropathic rat. Analgesia 1995; 1: 107-13.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55-63.
Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994; 59: 369-76.
Attal N, Jazat F, Kayser V, Guilbaud G. Further evidence for ‘pain-related’ behaviours in a model of unilateral peripheral mononeuropathy. Pain 1990; 41: 235-51.
Almasi R, Petho G, Bolcskei K, Szolcsanyi J. Effect of resiniferatoxin on the noxious heat threshold temperature in the rat: a novel heat allodynia model sensitive to analgesics. Br J Pharmacol 2003; 139: 49-58.
Bolcskei K, Helyes Z, Szabo A, et al. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain 2005; 117: 368-76.
Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol 1997; 121: 1513-22.
Bian F, Li Z, Offord J, et al. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res 2006; 1075: 68-80.
Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain 2003; 105: 133-41.
Jang JH, Nam TS, Paik KS, Leem JW. Involvement of peripherally released substance P and calcitonin gene-related peptide in mediating mechanical hyperalgesia in a traumatic neuropathy model of the rat. Neurosci Lett 2004; 360: 129-32.
Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett 1995; 197: 25-8.
Jang JH, Kim DW, Sang Nam T, Se Paik K, Leem JW. Peripheral glutamate receptors contribute to mechanical hyperalgesia in a neuropathic pain model of the rat. Neuroscience 2004; 128: 169-76.
Cunningham MO, Woodhall GL, Thompson SE, Dooley DJ, Jones RS. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur J Neurosci 2004; 20: 1566-76.
Field MJ, McCleary S, Hughes J, Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain 1999; 80: 391-8.
Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology 2006; 67: 1792-800.
Hamandi K, Sander JW. Pregabalin: a new antiepileptic drug for refractory epilepsy. Seizure 2006; 15: 73-8.
Knake S, Klein KM, Hattemer K, et al. Pregabalin-induced generalized myoclonic status epilepticus in patients with chronic pain. Epilepsy Behav 2007; 11: 471-3.
Huppertz HJ, Feuerstein TJ, Schulze-Bonhage A. Myoclonus in epilepsy patients with anticonvulsive add-on therapy with pregabalin. Epilepsia 2001; 42: 790-2.
Hellwig S, Amtage F. Pregabalin-induced cortical negative myoclonus in a patient with neuropathic pain. Epilepsy Behav 2008; 13: 418-20.
Murphy N, Mockler M, Ryder M, Ledwidge M, McDonald K. Decompensation of chronic heart failure associated with pregabalin in patients with neuropathic pain. J Card Fail 2007; 13: 227-9.
Pndage RL 2nd, Cantu M, Lindenfeld J, Hergott LJ, Lowes BD. Possible heart failure exacerbation associated with pregabalin: case discussion and literature review. J Cardiovasc Med (Hagerstown) 2008; 9: 922-5.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is accompanied by an editorial. Please see Can J Anesth 2010; 57(7).
Rights and permissions
About this article
Cite this article
Park, H.J., Joo, H.S., Chang, H.W. et al. Attenuation of neuropathy-induced allodynia following intraplantar injection of pregabalin. Can J Anesth/J Can Anesth 57, 664–671 (2010). https://doi.org/10.1007/s12630-010-9318-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-010-9318-0