Abstract
Purpose
Although intensive care unit (ICU) acquired sodium disturbances are common in critically ill patients, few studies have examined sodium disturbances in patients following cardiac surgery. The objective of this study was to describe the epidemiology of ICU-acquired hyponatremia and hypernatremia in patients following cardiac surgery.
Methods
We identified 6,727 adults (≥18 yr) who were admitted consecutively to a regional cardiovascular intensive care unit (CVICU) from January 1, 2000 to December 31, 2006 and were documented as having normal serum sodium levels (133 to 145 mmol·L−1) during the first day of ICU admission. ICU-acquired hyponatremia and hypernatremia were defined as a change in serum sodium concentration to <133 mmol·L−1 or >145 mmol·L−1, respectively, following ICU day one.
Results
A first episode of ICU-acquired hyponatremia and hypernatremia developed in 785 (12%) and 242 (4%) patients, respectively, (95% confidence interval [CI] 11-12% and 95% CI 3-4%, respectively), with a respective incidence density of 4.2 and 1.3 patients per 100 days of ICU admission (95% CI 4.0-4.5 and 95% CI 1.2-1.5). The incidence of ICU-acquired sodium disturbances varied according to the patients’ demographic and clinical variables for both hyponatremia (age, diabetes, Acute Physiology and Chronic Health Evaluation [APACHE II] score, mechanical ventilation, length of ICU stay, serum glucose level, and serum potassium level) and hypernatremia (APACHE II score, mechanical ventilation, length of hospital stay prior to ICU admission, length of ICU stay, serum glucose level, and serum potassium level). Compared with patients with normal serum sodium levels, hospital mortality was increased in patients with ICU-acquired hyponatremia (1.6% vs 10%, respectively; P < 0.001) and ICU-acquired hypernatremia (1.6% vs 14%, respectively; P < 0.001).
Conclusion
ICU-acquired hyponatremia and hypernatremia are common complications in critically ill patients following cardiac surgery. They are associated with patient demographic and clinical characteristics and an increased risk of hospital mortality.
Résumé
Objectif
Bien que les désordres sodiques acquis à l’unité des soins intensifs (USI) soient fréquents chez les patients gravement malades, peu d’études ont examiné les désordres sodiques chez les patients après une chirurgie cardiaque. L’objectif de cette étude était de décrire l’épidémiologie de l’hyponatrémie et de l’hypernatrémie acquises à l’USI après une chirurgie cardiaque.
Methode
Nous avons identifié 6727 adultes (≥ 18 ans) admis consécutivement dans une unité de soins intensifs cardiovasculaires régionale (USICV) entre le 1er janvier 2000 et le 31 décembre 2006, et chez lesquels des niveaux normaux de sodium sérique (133 à 145 mmol·L−1) pendant leur première journée d’admission à l’USI avaient été documentés. L’hyponatrémie et l’hypernatrémie acquises à l’USI ont été définies comme un changement de la concentration de sodium sérique à < 133 mmol·L−1 ou > 145 mmol·L−1, respectivement, après le jour 1 à l’USI.
Résultats
Un premier épisode d’hyponatrémie ou d’hypernatrémie acquises à l’USI est apparu chez 785 (12 %) et 242 (4 %) des patients, respectivement (intervalle de confiance [IC] 95 %, 11-12 % et IC 95 %, 3-4 %, respectivement), avec une densité d’incidence respective de 4,2 et 1,3 patients par 100 jours d’admission à l’USI (IC 95 %, 4,0-4,5 et IC 95 %, 1,2-1,5). L’incidence des désordres sodiques acquis à l’USI a varié selon les variables démographiques et cliniques des patients, aussi bien pour l’hyponatrémie (âge, diabète, score APACHE II (Acute Physiology and Chronic Health Evaluation), ventilation mécanique, durée de séjour à l’USI, niveau de glucose sérique, et niveau de potassium sérique) que pour l’hypernatrémie (score APACHE II, ventilation mécanique, durée de séjour à l’hôpital avec l’admission à l’USI, durée de séjour à l’USI, niveau de glucose sérique et niveau de potassium sérique). Par rapport aux patients présentant des niveaux normaux de sodium sérique, la mortalité hospitalière était plus élevée chez les patients souffrant d’hyponatrémie acquise à l’USI (1,6 % vs 10 %, respectivement; P < 0,001) et d’hypernatrémie acquise à l’USI (1,6 % vs 14 %, respectivement; P < 0,001).
Conclusion
L’hyponatrémie et l’hypernatrémie acquises à l’USI sont des complications fréquentes chez les patients gravement malades après une chirurgie cardiaque. Elles sont associées aux caractéristiques démographiques et cliniques des patients ainsi qu’à un risque accru de mortalité hospitalière.
Similar content being viewed by others
The sodium disturbances, hyponatremia and hypernatremia, are among the laboratory abnormalities detected most frequently in patients admitted to intensive care.1 They are important markers of a critically ill patient’s clinical status that often prompt changes to a patient’s treatment, and they are associated with an increased risk of death.2 Previous studies have suggested that intensive care unit (ICU) acquired sodium disturbances are largely preventable and have been proposed as quality of care indicators.3 The epidemiology of sodium disturbances in critically ill medical, surgical, trauma, and neurosurgical patients has been evaluated.3 - 6
However, there is little information on the incidence, clinical setting, and outcome of sodium disturbances, as they occur in a large unselected population of critically ill patients following cardiac surgery. Most of the available information is focused on general electrolyte disturbances and is based on case reports7 or small cohort studies.8 , 9 Therefore, we undertook a study of unselected patients admitted to a regional cardiovascular intensive care unit (CVICU) over a seven-year period to examine the epidemiology of ICU-acquired hyponatremia and hypernatremia in patients following cardiac surgery.
Methods
Study questions
The primary objective of our study was to examine the epidemiology of ICU-acquired hyponatremia and hypernatremia in patients following cardiac surgery. We asked three specific questions for patients admitted to a CVICU following cardiac surgery.
-
1.
What is the incidence of ICU-acquired hyponatremia and hypernatremia?
-
2.
What demographic and clinical patient factors are independently associated with ICU-acquired hyponatremia and hypernatremia? and
-
3.
What is the hospital mortality for patients who develop ICU-acquired hyponatremia or hypernatremia compared with patients who maintain normal serum sodium levels during their ICU stay?
Study population
The Calgary Health Region (CHR) administers all publicly funded hospital care to the residents of the cities of Calgary and Airdrie and approximately 20 nearby towns (population 1.2 million) in the province of Alberta, Canada.10 All adult patients in the CHR who require cardiac surgery are managed in a 12-bed CVICU under the care of the Department of Critical Care Medicine, University of Calgary and the CHR. This ICU is a closed unit staffed by fully trained intensivists.
For this study, we utilized a population-based inception cohort design. We identified adults (≥ 18 yr) who were admitted consecutively to the CVICU from January 1, 2000 to December 31, 2006 and underwent cardiac surgery (maximum data warehouse sample size at time of ethics submission). If patients were admitted to the CVICU more than once during the study period, we selected only their first CVICU admission for review. Patients were included in the study cohort if their CVICU stay was longer than one calendar day in duration and if they were documented as having exclusively “normal” serum sodium level(s) as per the Calgary Laboratory Services (CLS) reference range (133 to 145 mmol·L−1) during the first calendar day of their CVICU admission.11 Patients with pre-existing dialysis dependence or those who received renal replacement therapy on CVICU admission day one were excluded. The Conjoint Health Research Ethics Board at the University of Calgary and the CHR approved this study and waived the need for informed consent from patients.
Data
Demographic, hospital, and clinical data were obtained using the regional CVICU patient data warehouse, TRACER. TRACER is a clinical warehouse that stores longitudinal information on all patients admitted to CVICU in the CHR. Data sources include an electronic patient information system, Quantitative Sentinel (QS) (GE-Marquette Medical Systems Inc. Milwaukee, WI, USA), interfaced to all bedside monitoring and ventilator devices that capture physiological and ventilation data. These data are validated (accepted by the system) by nursing or respiratory therapy staff on at least an hourly basis by examining the degree to which they are representative and plausible. An HL-7 interface with the regional laboratory information system, Cerner PathNet Classic version 306 (Kansas City, MO, USA), is utilized to collect laboratory data. Basic laboratory data, e.g., complete blood count, electrolytes, and renal function, are collected on patients in the ICU each morning, with additional laboratory values collected at the discretion of the clinicians. The most abnormal (maximum and minimum) physiologic and laboratory values in each 24-hr period (00:00-23:59 hr) are exported to the data warehouse. For analysis purposes, the value deviating furthest from the median of the reference range was taken, and the maximum value was taken where there was no difference between the minimum and maximum value from the median. A sensitivity analysis was performed using the minimum value which produced similar results. Administrative information, including age, gender, location of residence, date of admission, date of discharge, and discharge status are routinely imported from the regional corporate database.
Patient characteristics
Patient characteristics were classified a priori into time-independent and time-dependent factors. Time-independent factors included demographic (age, sex, diabetes, baseline renal dysfunction), hospital (weekend admission, night admission), and clinical (type of surgery, urgency of surgery, admission APACHE II score, and admission Therapeutic Intervention Scoring System [TISS] score) characteristics. Time-dependent patient factors included vital signs, Glasgow coma score, all laboratory values, and level of care (full care, full care without cardiopulmonary resuscitation, and comfort care). Severity of illness at inception, i.e., within the first day of ICU admission, was assessed using the APACHE II and TISS scores.2 , 12 Hyponatremia was defined as a serum sodium concentration <133 mmol·L−1. Hypernatremia was defined as a serum sodium concentration >145 mmol·L−1. Patients were classified as experiencing multiple distinct sodium disturbances if abnormal serum sodium measurements (<133 mmol·L−1 or >145 mmol·L−1) were separated by a minimum of one calendar day of normal serum sodium measurements (133-145 mmol·L−1). Baseline renal dysfunction was defined as a creatinine level >100 μmol·L−1 during the first day of ICU admission (Calgary Laboratory Services reference range <100 μmol·L−1 for adult females).11 Diabetes was defined as a glucose level ≥11.1 mmol·L−1.13 A normal serum concentration of potassium was defined as 3.5-5.0 mmol·L−1.
Statistical analysis
The strategy for the primary analysis was to answer each of the three specific study questions using event-based analyses to take advantage of all of the available information in our longitudinal data.14 , 15 Data were summarized initially with means, median, standard deviations, and interquartile ranges for continuous variables and frequencies for categorical variables. In order to make univariable comparisons between normal, hyponatremic, and hypernatremic subgroups, Pearson’s Chi square tests were used for categorical variables and analysis of variance, and Kruskal-Wallis tests were used for continuous variables. Missing lab values (3.8% of patient ICU days had no serum sodium value recorded) were imputed with the value on the closest previous or following day, where available, within a 48 hr window. A complete case analysis produced similar findings. Multivariable models for ICU-acquired hyponatremia and hypernatremia were determined using generalized estimating equations with a logit link in order to adjust for repeated measures. A first-order autoregressive correlation structure was assumed for both models due to the longitudinal nature of the data. Models for single outcome measures were formulated using logistic regression for binary measures (renal replacement therapy and mortality) and linear regression for continuous measures (duration of vasopressor use, duration of ventilation, and length of stay). Various transformations of continuous variables were assessed in order to select the best functional form to meet the assumptions of linearity. For each model, backward selection was used to evaluate candidate variables with P values < 0.1 from the univariable analyses to find the most parsimonious model. To evaluate goodness of fit and assist model selection, the Akaike’s information criterion (AIC) and the Hosmer-Lemeshow statistic were calculated for logistic regression models. Linear regression models were assessed using residual analysis. The quasilikelihood under the independence model criterion (QIC) statistic developed by Pan was used to assess the correlation structure and to compare model fit of generalized estimating equations.16 All results were calculated using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA), and a significance level of 0.05 was used for all analyses.
Results
Baseline data
During the seven-year study period, 7,957 patients were admitted to the CVICU. We excluded 81 patients with an ICU stay of less than one day duration and 301 patients with either pre-existing dialysis dependence or who received renal replacement therapy on the first day of ICU admission. Among the remaining 7,555 adults, 6,727 (89%) were documented to have normal serum sodium levels during their first day of ICU admission. The baseline characteristics of the study population (n = 6,727) are summarized in Table 1. Seventy-six percent (n = 5,142) of the patients were male; the median age was 66.8 (interquartile range [IQR], 57.8-73.9) yr, and the mean APACHE II score at first admission was 25.1 (standard deviation [SD] 5.1). Of the ICU admissions, 4,984 (74%) were classified as elective surgeries, predominantly for coronary artery bypass grafting (n = 3,721) (74%). The mean serum sodium value for patients during their first day of ICU admission was 139.4 mmol·L−1 (SD 3.1 mmol·L−1).
Incidence
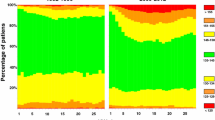
Among the 6,727 patients with normal serum sodium levels during their first day of ICU admission, a first episode of ICU-acquired hyponatremia developed in 785 (12%) patients and hypernatremia in 242 (4%) patients (95% CI 11-12% and 95% CI 3-4%, respectively). The proportion of ICU patients with ICU-acquired hyponatremia and hypernatremia during the first 30 days of ICU stay is summarized in Figure 1. Based on the 22,945 ICU admission days, the incidence density for a first episode of ICU-acquired hyponatremia and hypernatremia was 4.2 (95% CI 4.0-4.5) and 1.3 (95% CI 1.2-1.5) per 100 days of ICU admission, respectively. The median time from ICU admission to patients developing an ICU-acquired sodium disturbance was two (IQR 1-3) days for hyponatremia and one (IQR 1-3) day for hypernatremia. The median rate of change in serum sodium levels was 2 (IQR 1-4) mmol·L−1·day−1 for patients developing a first episode of hyponatremia and 1.5 (IQR 0.5-3.0) mmol·L−1·day−1 for hypernatremia (P = 0.017). Ninety patients experienced a change in serum sodium levels ≥ 12 mmol·L−1·day−1; 63 (8.0%) patients with ICU-acquired hyponatremia, 26 (10.7%) patients with ICU-acquired hypernatremia, and one patient (0.02%) who remained within the reference range. Twelve percent of the patients with a sodium disturbance experienced more than one distinct sodium disturbance during their ICU stay. Ten percent (n = 77) of patients with ICU-acquired hyponatremia experienced more than one episode of hyponatremia compared with 12% (n = 29) of patients with ICU-acquired hypernatremia who experienced more than one episode of hypernatremia (P = 0.39). Thirty-seven patients (3.6% of patients with ICU-acquired sodium disturbances) experienced distinct episodes of both hyponatremia and hypernatremia during their ICU stay. The mean serum sodium levels for patients during episodes of ICU-acquired hyponatremia and hypernatremia were 130 mmol·L−1 (SD 2.6 mmol·L−1) and 149 mmol·L−1 (SD 3.4 mmol·L−1), respectively. Among patients with sodium disturbances, the median number of days of hyponatremia and hypernatremia was two (IQR 1-2 days).
Patient characteristics
The incidence of ICU-acquired hyponatremia and ICU-acquired hypernatremia varied according to patient characteristics (Table 2). Patients with higher APACHE II scores, longer ICU stays, and higher serum glucose levels were at greater risk of both ICU-acquired hyponatremia and hypernatremia. Serum potassium disturbances were associated with both sodium disturbances. Mechanical ventilation was associated with a decreased risk of ICU-acquired hyponatremia but was associated with an increased risk of ICU-acquired hypernatremia. Age and diabetes were associated with ICU-acquired hyponatremia, while duration of hospital stay before ICU admission was associated with ICU-acquired hypernatremia.
Mortality
The overall ICU and hospital mortality rates for the study population were 1.7% and 3.1%, respectively. Compared with patients with normal serum sodium levels, mortality was increased for patients with ICU-acquired hyponatremia and hypernatremia (Table 3). A dose response relationship was observed for the magnitude of the ICU-acquired sodium disturbance (absolute deviation from normal range) for both ICU (P < 0.001) and hospital (P < 0.001) mortality (Figure 2). Compared with patients with lower rates of change, patients with a documented rate of change in serum sodium levels of ≥ 12 mmol·L−1·day−1 during their ICU stay had higher ICU (28.2% vs 1.3%; P < 0.001) and hospital mortality (31.8% vs 2.7%; P < 0.001).
Discussion
This study is important because it is the first epidemiological evaluation of ICU-acquired sodium disturbances in a large unselected population of patients following cardiac surgery. The results demonstrate that ICU-acquired hyponatremia and hypernatremia are common in critically ill patients following cardiac surgery. The occurrence of ICU-acquired hyponatremia and hypernatremia varies significantly among patients with different demographic and clinical characteristics. There is a strong association between both ICU-acquired hyponatremia and hypernatremia and in-hospital patient mortality. Despite serum sodium levels being among the most frequently measured laboratory values, the epidemiology of sodium disturbances in patients following cardiac surgery has been the focus of only a small number of epidemiologic studies.8 , 9
Acute hyponatremia is a recognized complication of cardiac surgery. Chung et al. identified that 12 out of 52 (23%) patients developed postoperative hyponatremia (defined as a sodium < 130 mmol·L−1) within one week of their cardiovascular operation. Among their larger cohort of postoperative hyponatremic patients that included general surgical, orthopedic, and transplant patients, the most common clinical settings of hyponatremia were normovolemic states, edematous states, and hyperglycemia. Ninety-four percent of patients were receiving hypotonic fluids at the onset of hyponatremia. Polderman et al. studied 500 patients following cardiac surgery and reported hyponatremia (defined as a sodium < 135 mmol·L−1) in 6% of patients on admission to ICU.9 These electolyte disturbances were attributed to a combination of increased urinary excretion and intracellular shift induced by a combination of intraoperative hypothermia and extracoporeal circulation.9 , 17 Both studies provided important contributions to the literature, examined hyponatremia, but did not evaluate potential associations between hyponatremia and patient characteristics or outcomes.
In contrast, our study evaluated the incidence, associated factors, and outcomes of both ICU-acquired hyponatremia and hypernatremia in patients following cardiac surgery. The results demonstrate that ICU-acquired hyponatremia and hypernatremia are common; their incidence varies significantly among patients with different demographic and clinical characteristics, and they are associated with an increased risk of hospital mortality. Compared with other critically ill populations, a novel finding is that patients are more likely to develop ICU-acquired hyponatremia than hypernatremia following cardiac surgery.4 The risk of developing ICU-acquired hyponatremia increases rapidly during the first few days of ICU stay before gradually decreasing. This risk contrasts with the risk of developing ICU-acquired hypernatremia, wherein the incidence steadily increases over time. These differing patterns of sodium disturbance may be secondary to many potential etiologies, including perioperative fluid shifts, changing levels of antidiuretic hormone activity, new onset congestive heart failure, and diuresis. An important finding is that a strong association exists between the magnitude of ICU-acquired sodium disturbances and hospital mortality. The dose response relationship between sodium deviation and hospital mortality highlights that even small deviations in serum sodium concentration from the normal range are associated with increased mortality.
Our study adds to the growing body of literature highlighting the importance of sodium disturbances in critically ill patients. In a retrospective one-year study from a Dutch Medical ICU, Polderman et al. reported hypernatremia (defined as a sodium ≥ 150 mmol·L−1) in 9% of patients admitted to the ICU, with an additional 6% of patients developing hypernatremia during their ICU stay.3 Patients who presented with hypernatremia had a 20% hospital mortality rate compared with 32% of patients who acquired hypernatremia during their ICU stay.3 Lindner et al. described a similar incidence of hypernatremia in a medical ICU in Austria, but they reported higher hospital mortality for patients presenting with hypernatremia than those acquiring the disorder (43% vs 39%, respectively).5 Similarly, in a retrospective five-year review of a medical ICU in France, Bennani reported a 14% incidence of hyponatremia (defined as a sodium < 130 mmol·L−1), with severe hyponatremia (defined as a sodium < 125 mmol·L−1) associated with increased mortality.6 In an earlier study of three medical-surgical ICUs, we demonstrated that a first episode of ICU-acquired hyponatremia developed in 11% of patients, and ICU-acquired hypernatremia developed in 26% of patients.4 Compared with patients with normal serum sodium levels, hospital mortality increased in patients with ICU-acquired hyponatremia (16% vs 28%, respectively; P < 0.001) and increased in patients with ICU-acquired hypernatremia (16% vs 34%, respectively; P < 0.001). Finally, sodium disturbances are important in patients with heart disease. Hyponatremia has a reported incidence of 20-30% in patients hospitalized for worsening heart failure, and it is associated with increased morbidity and mortality.18 - 20 Whether they are diagnosed with medical, surgical, or cardiac disease, critically ill patients appear to be at high risk of developing sodium disturbances wherein the incidence of hyponatremia varies according to patient demographic and clinical characteristics and is associated with an increased risk of mortality.
There are several limitations of our study. First, this is a retrospective analysis of a clinical data source that captures detailed demographic, hospital, physiological, and laboratory data. Confounding factors that could have influenced the relationship between serum sodium concentration and outcomes, such as parenteral fluids and medication use, may have been present and not accounted for in this analysis. Second, we can only detect associations from our data and not causality. Thus, we cannot determine whether ICU-acquired sodium disturbances are a marker of illness severity, an adverse outcome, or a reflection of the pathophysiology of recovery from cardiac surgery. Similarly, one cannot infer from the results of our study that correction of sodium disturbances will result in improved patient outcomes. Third, our results are based on patients admitted to a single CVICU serving a population of 1.2 million people. Although our data are based on a large unselected population of patients undergoing cardiac surgery, it is possible that patients treated in other types of ICUs or in other health regions may have different experiences.
In conclusion, this large study conducted in a broad non-selected population of adult patients demonstrates that ICU-acquired hyponatremia and hypernatremia are common following cardiac surgery. In addition, patients with higher illness severity, longer lengths of ICU stay, higher serum glucose levels, and abnormal serum potassium levels are at risk for sodium disturbances. The role of electrolyte monitoring is uncertain, and clinical trials investigating management strategies are warranted.
References
Arieff AI. Acid-base, electrolyte, and metabolic abnormalities. In: Parrillo JE, Dellinger RP (Eds). Critical Care Medicine: Principles of Diagnosis and Management in the Adult, 2nded. St. Louis: Mosby; 2002.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818-29.
Polderman KH, Schreuder WO, Strack van Schijndel RJ, Thijs LG. Hypernatremia in the intensive care unit: an indicator of quality of care? Crit Care Med 1999; 27: 1105-8.
Stelfox HT, Ahmed SB, Khandwala F, Zygun D, Shahpori R, Laupland K. The epidemiology of intensive care unit-acquired hyponatraemia and hypernatraemia in medical-surgical intensive care units. Crit Care 2008; 12: R162.
Lindner G, Funk GC, Schwarz C, et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis 2007; 50: 952-7.
Bennani SL, Abouqal R, Zeggwagh AA, et al. Incidence, causes and prognostic factors of hyponatremia in intensive care (French). Rev Med Interne 2003; 24: 224-9.
Fitzsimons MG, Agnihotri AK. Hyponatremia and cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2007; 21: 273-5.
Chung HM, Kluge R, Schrier RW, Anderson RJ. Postoperative hyponatremia, A prospective study. Arch Intern Med 1986; 146: 333-6.
Polderman KH, Girbes AR. Severe electrolyte disorders following cardiac surgery: a prospective controlled observational study. Crit Care 2004; 8: R459-66.
Anonymous. Alberta Registry Population at March 2005 (December 2003 RHA Boundaries) Available from URL: http://www.crha-health.ab.ca/qshi/hsau/Demographic_Data/RHA_registry_population_1999_2005.xls (accessed February 2010).
Alberta Health Services. Directory of Tests. Available from URL: http://www.calgarylabservices.com/LabTests/ (accessed February 2010).
Cullen DJ, Civetta JM, Briggs BA, Ferrara LC. Therapeutic intervention scoring system: a method for quantitative comparison of patient care. Crit Care Med 1974; 2: 57-60.
Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Canadian Journal of Diabetes 2008; 32 (Suppl 1): iv-S201.
Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986; 42: 121-30.
Lipsitz SR, Fitzmaurice GM, Orav EJ, Laird NM. Performance of generalized estimating equations in practical situations. Biometrics 1994; 50: 270-8.
Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics 2001; 57: 120-5.
Polderman KH, Peerdeman SM, Girbes AR. Hypophosphatemia and hypomagnesemia induced by cooling in patients with severe head injury. J Neurosurg 2001; 94: 697-705.
Gheorghiade M, Abraham WT, Albert NM, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J 2007; 28: 980-8.
Gheorghiade M, Rossi JS, Cotts W, et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med 2007; 167: 1998-2005.
Klein L, O’Connor CM, Leimberger JD, et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation 2005; 111: 2454-60.
Funding sources
No external funding was received for this study. H.T. Stelfox is supported by a New Investigator award from the Canadian Institutes of Health Research. None of the authors has financial or professional conflicts of interest that would influence the conduct or reporting of this study. H.T. Stelfox had full access to all of the study data and assumes responsibility for the integrity of the data and the accuracy of the analysis.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article will be accompanied by an editorial. Please see Can J Anesth 2010; 57(7).
Appendix
Rights and permissions
About this article
Cite this article
Stelfox, H.T., Ahmed, S.B., Zygun, D. et al. Characterization of intensive care unit acquired hyponatremia and hypernatremia following cardiac surgery. Can J Anesth/J Can Anesth 57, 650–658 (2010). https://doi.org/10.1007/s12630-010-9309-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-010-9309-1