Abstract
Purpose
We tested the hypothesis that the development of sympathectomy-mediated vasodilatation is dependent on the concentration rather than the dose of epidural local anesthetic administered.
Methods
Sixty subjects receiving lumbar epidural bupivacaine were randomised to one of three groups: A: 10 ml 0.5% (50 mg); B: 10 ml 0.25% (25 mg); and C: 40 ml 0.0625% (25 mg). Groups A and B had equal volume but a twofold difference in drug dose, while groups B and C had equal drug dose, but a fourfold difference in drug volume. At baseline and 5, 10, and 20 min following epidural bupivacaine administration, we assessed the following indices of sympathectomy: pulse oximeter perfusion index in the toe and finger, skin temperature in the toe and finger, and mean arterial pressure. We also assessed sensory level (pinprick, cold, and light touch) and motor block.
Results
There was an increase in the pulse oximeter perfusion index by 20 min of 280%, 303%, and 59% in groups A, B, and, C, respectively. There was a significant sympathectomy-mediated vasodilatation in the toe for both groups A (P = 0.002) and B (P < 0.001) but not C (P = 0.22). Vasoconstriction in the finger was observed in group A only (P = 0.015) but not in group B (P = 0.09) or group C (P = 0.20). There were similar blood pressure changes and similar sensory changes in all groups. The intensity of motor block increased with increasing drug concentration.
Conclusions
Our observations suggest that drug concentration is more important than drug dose in determining the degree of sympathectomy following lumbar epidural local anesthesia.
Résumé
Objectif
Nous avons testé l’hypothèse selon laquelle l’apparition d’une vasodilatation médiée par sympathectomie était liée à la concentration plutôt qu’à la dose d’anesthésique local péridural administré.
Méthode
Soixante patients recevant de la bupivacaïne péridurale lombaire ont été randomisés en trois groupes : A : 10 ml 0,5 % (50 mg); B : 10 ml 0,25 % (25 mg); et C : 40 ml 0,0625 % (25 mg). Les groupes A et B ont reçu un volume équivalent mais la dose de médicament était doublée pour le groupe A, alors que les groupes B et C ont reçu une dose égale, mais le volume de médicament a été quadruplé pour le groupe C. Nous avons évalué les indices de sympathectomie suivants aux niveaux de base ainsi qu’à 5, 10 et 20 minutes après l’administration de la bupivacaïne péridurale : indice de perfusion de l’oxymètre de pouls à l’orteil et au doigt, température cutanée à l’orteil et au doigt, et tension artérielle moyenne. Nous avons également évalué le niveau sensoriel (piqûre à l’aiguille, froid et toucher léger) et le bloc moteur.
Résultats
Sur la base de l’indice de perfusion de l’oxymètre de pouls, nous avons observé une vasodilatation médiée par sympathectomie significative dans l’orteil pour les groupes A (P = 0,002) et B (P < 0,001) mais pas dans le groupe C (P = 0,22). La vasoconstriction au doigt a été observée uniquement dans le groupe A (P = 0,015), et non dans les groupes B (P = 0,09) ou C (P = 0,20). Des changements semblables au niveau de la pression artérielle et des changements sensitifs semblables ont été observés dans tous les groupes. L’intensité du bloc moteur a augmenté avec l’augmentation de la concentration de médicament.
Conclusion
Nos observations suggèrent que la concentration de médicament joue un rôle plus important que la dose de médicament pour déterminer le niveau de sympathectomie à la suite d’une anesthésie locale péridurale lombaire.
Similar content being viewed by others
Introduction
The factors that determine the distribution of local anesthetic effects in the epidural space and the intensity and extent of their action are still the subject of scientific debate. The predominant factors studied have been the inter-related triad of drug dose, volume, and concentration.
Over the past two decades, facilitated by the co-administration of epidural opioids, there has been a dramatic change in the use of epidural local anesthetics in labor and postoperative pain management, with a reduction in total drug dose and a disproportionately far greater reduction in drug concentration.1 However, data from clinical research are divided, with multiple studies reporting that the dose of epidural local anesthetic is the most important factor in determining the extent of differential sensory block, irrespective of its volume of dilution.2–12
Accordingly, we designed a study to test the hypothesis that the development of sympathectomy-mediated vasodilatation in the toe is dependent on the concentration of epidural local anesthetic rather than on the total dose of drug administered.
Methods
We designed a prospective randomized double-blind trial of three different preparations of epidural bupivacaine; group A: 10 ml 0.5% (50 mg), group B: 10 ml 0.25% (25 mg), and group C: 40 ml 0.0625% (25 mg). Groups A and B had equal volumes of dilution but a twofold difference in drug dose, while groups B and C had equal drug dose with a fourfold difference in volume of dilution.
Selection and description of participants
The Institutional Review Board of Hadassah Hebrew University Medical Center approved this research study. Sixty patients were enrolled for this study after signing informed consent. All patients were scheduled for water-immersed extracorporeal shock wave lithotripsy (Dornier HM3, Dornier MedTech Europe, Munich, Germany) under epidural anesthesia. Exclusion criteria included: ASA physical status III or higher, age younger than 18 or older than 70 years, peripheral vascular disease, diabetes, baseline neurological deficit, or any contraindication for regional anesthesia.
The patients were enrolled by a physician who was blinded to the group allocation. Computer-generated random numbers were used to allocate the subjects to their study groups. The allocation, which was concealed by using serial-numbered opaque envelopes, took place following placement of the epidural catheter. A nonblinded anesthesiologist performed the drug preparation and administration; however, the patients were blinded to their treatment groups, as was the investigator who recorded the clinical data.
Technical information
The study was conducted in the anesthesia holding area of the lithotripsy suite where the ambient temperature was controlled at 24°C. It should be noted that the study was completed before the commencement of lithotripsy. Immediately prior to epidural placement, the subjects received 1000 ml of intravenous lactated Ringer’s solution at room temperature, and they were placed in the lateral decubitus position for epidural placement. Using a midline approach, a 17-G Tuohy needle was inserted into the epidural space at the L2/3 or L3/4 interspace. The epidural space was identified by loss of resistance to air. An 18-G multi-orifice epidural catheter was inserted approximately 4 cm into the epidural space, and a negative pressure aspiration test was performed. Following placement of the epidural and before the anesthesiologist dosed the catheter, the subjects rested for 20 min before baseline data were collected. The subjects reclined throughout the study period without sedation. They were covered with a thin cotton sheet, but their legs were exposed above the knee level in order to allow them to equilibrate with ambient temperature.
Epidural drugs were administered through the epidural catheter as incremental doses over 5 min; time intervals were reported from the completion of drug administration. Data were measured for a 20-min period. Following completion of the study, the patients received additional epidural local anesthetic medication as needed for the lithotripsy procedure. The adequacy of the epidural analgesia for the subsequent ESWL procedure was noted; there was no long-term follow-up.
Measurements: The following measurements were recorded at baseline and at 5, 10, and 20 min following epidural drug administration: indices of sympathectomy, sensory level (pinprick, cold, and light touch), and motor power (modified Bromage score).
Indices of sympathectomy
Study endpoints were quantitative photoplethysmography (PPG) waveform analysis, skin temperature recording, and blood pressure monitoring.
We used quantitative PPG waveform analysis as our primary assessment of sympathectomy in this study; the methodology for PPG waveform analysis has been described in detail in recent comprehensive reviews.13,14 Briefly, PPG is an optical technique that measures the absorption of light by biological tissue. The PPG waveform consists of a pulsatile “AC” signal superimposed on a nonpulsatile baseline “DC” signal (Fig. 1). The pulse oximeter perfusion index (PI)15,16 is the ratio of the amplitude of the “AC” signal to the baseline “DC” signal. The PI has been correlated with changes in vascular sympathetic tone17,18 and blood volume13,14 and has been validated with assessments of perfusion made by near-infra-red spectroscopy.16 Previous reports from our group suggest that the PI is a more reliable and a more sensitive marker of sympathectomy-related vasodilatation than either blood pressure changes or cutaneous temperature changes.18,19
The photoplethysmograph (PPG) waveform. AM represents the amplitude of the pulsatile “AC” signal, and BL represents the baseline or the nonpulsatile “DC” signal. The pulse oximeter perfusion index (PI) is the AM/BL ratio. Note that the PPG waveform in commercially available devices is typically inverted so as to make the PPG waveform resemble the arterial pressure waveform
The PPG sensor in this study was a pulse oximetry sensor probe (N-25 oxisensor II, Nelcor Puritan Bennett Inc., Pleasanton, CA, USA), wherein we only used the infrared light source and the photo detector. The PPG waveform was measured on the patient’s second toe and on the index finger. A 2-min section of PPG pulses at the toe and at the finger was recorded at each observation time point, during which time the patient was asked to refrain from any movement. PPG waveforms from each of the 2-min examinations were digitally analyzed by a blinded technician, and a section with 60 consecutive pulses displaying low baseline artefact was chosen for subsequent quantitative analysis. The technician was blinded to the following: (1) clinical data, (2) the site of the data recording (toe or finger), and (3) the time of data recording (baseline or 5, 10, or 20 min following epidural anesthesia). As reported previously, the baseline (BL) and amplitude (AM) signal was digitally identified, quantified, and averaged over successive pulsations for each individual pulse in the PPG waveform tracing.20–22 It should be noted that PPG waveform analysis was performed “off-line” after completion of the study; thus, if baseline or movement artefact was noted, it was not possible to improve data signal quality. In these cases, the PPG waveform was not accepted for analysis. The change in the PI from baseline, in both the toe and the finger, was expressed at each time interval as incremental change from baseline (dPI). With respect to baseline, positive change represents vasodilatation; negative change represents vasoconstriction.
Cutaneous temperature was measured with thermocouples (AH2 Ellab Instruments, Copenhagen, Denmark) applied to the big toe, thigh, upper arm, and thumb. The thermocouples were connected to a multi-channel body temperature monitoring and data acquisition system. Skin temperature (T) was recorded in degrees centigrade (°C), as were the gradients between the skin temperature of the leg and the toe (Tleg-toe) and the arm and the finger (Tarm-finger). The change in skin temperature at the toe and the finger and the change in the leg-toe and arm-finger temperature gradients were expressed as incremental changes from baseline (dTtoe, dTfinger, dTleg-toe, and dTarm-finger, respectively).
The mean arterial pressure (MAP) was assessed using noninvasive blood pressure measured in the forearm. The change in MAP from baseline was expressed as incremental change from baseline (dMAP).
The upper sensory levels to pinprick, cold, and light touch were assessed by the blinded observer. To maximize consistency, all data were recorded by the same observer, and data were recorded with reference to a poster of the skin dermatomes that was placed over the subject’s bed. Pinprick was assessed using the light application of a 23G needle; cold was tested using ethylglycol spray, and light touch was tested using a blunt wooden applicator. We arbitrarily designated a numerical code to the dermatomes as numbers above the space of epidural insertion; thus, L4 = −1, L3 = 0, L2 = +1, etc. The lower sensory level was not assessed.
Motor block was assessed using a modified Bromage score (0 = no motor block, 1 = unable to straight leg raise, 2 = unable to flex knee against resistance, 3 = unable to flex ankle).
Statistical considerations
We examined the effect of drug volume, dose, and concentration on indices of sympathectomy, sensory level, and motor block. The a priori primary end-point was the incremental change in the pulse oximeter PI in the toe and the finger at 20 min from drug administration.
Baseline data were compared between the three study groups with the Krushkal Wallis test. Baseline and outcome data were compared between the PPG accepted and rejected subjects with the Mann–Whitney U-test.
Drug effect was assessed using repeated measures analysis of variance (RM-ANOVA) with simple contrast referencing baseline; the conservative Greenhouse–Geisser modification was used if sphericity assumptions were not met. The effects of dose and volume were assessed using repeated measures ANOVA by selecting these variables as independent between-subject factors for pooled data. Pair-wise post hoc multiple comparisons were used with Bonferonni’s correction to test for differences between the three group concentrations at 20 min. The effect of drug concentration on motor block was assessed using the Pearson chi-squared test.
Based on published variance in the pulse oximeter PI21 (standard deviation of 0.87), the use of a two-tailed one-way ANOVA with three groups, a power of 0.9, and an alpha error of 0.05, we calculated that 20 patients were needed in each group to detect a 40% difference between groups in the dPItoe at 20 min. Sample size calculations were performed using Power and Precision (Biostat Inc, Englewood, NJ, USA).
Statistical analysis was performed using SPSS version 10.0 or higher (SPSS Inc., Chicago, IL, USA). Statistical significance was assumed at P ≤ 0.05.
Results
Subjects and demographics
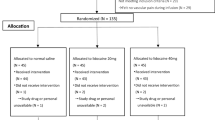
Sixty subjects were initially enrolled for this study (Table 1 shows demographic and baseline data between groups).
After completion of the study, it was found that 14 patients had PPG data that did not meet a priori criteria for signal data quality (Fig. 2). Poor signal quality was not identified at the time of the study, as the PPG signals were analyzed “off-line.” All patients had full data recorded for cutaneous temperature changes, blood pressure, and sensory motor block. We compared the patients with adequate PPG signals and those who were rejected because of inadequate PPG signals. There were no clinically relevant or statistically significant differences for either baseline data (age; P = 0.84, sex; P = 0.29, baseline mean arterial pressure; P = 0.36, baseline heart rate; P = 0.58, baseline toe temperature; P = 0.46, baseline finger temperature; P = 0.27) or outcome data (20 min mean arterial pressure; P = 0.92, 20 min toe temperature; P = 0.14, 20 min finger temperature; P = 0.28) (Table 2). Only those patients for whom there were adequate PPG data had their PPG data presented graphically and included in the primary statistical analysis.
Correct epidural catheter placement was verified in all patients by the addition of further doses of epidural local anesthetic sufficient to cause bilateral sensory block, which allowed the subsequent lithotripsy procedure to be performed without pain. There were no cases of epidural catheter failure.
Sympathectomy
The dPItoe, dPIfinger, dTtoe, dTfinger, and dMAP over time are shown in Fig. 3. To summarize these changes, there was a marked vasodilatation of the toe in group A (10 ml 0.5% bupivacaine) and group B (10 ml 0.25% bupivacaine), but not in group C (40 ml 0.0625% bupivacaine). Group A experienced a significant compensatory vasoconstriction in the finger but not in groups B or C. There were similar blood pressure changes in all groups. The degree of sympathectomy-mediated vasodilatation in the foot was more pronounced after epidural administration of high concentration low volume local anesthetics than after low concentration high volume solutions of equal dose.
Indices of sympathetic-mediated vasodilatation following lumbar epidural anesthesia with epidural bupivacaine. The upper two graphs represent the incremental change from baseline in pulse oximeter perfusion index (dPI) (Fig. 2a) and the skin temperature (Fig. 2b), respectively. Gray circles represent measurements at the toe; white circles represent measurements at the finger. Positive change with respect to baseline represents vasodilatation; negative change represents vasoconstriction. The lower graph represents change in MAP (Fig. 2c). Data are presented against time from epidural drug administration. Points marked with an asterisk (*) represent statistically significant change from baseline (see text)
There was a significant increase in the pulse oximeter PI over time in the toe for groups A and B (P = 0.002 and P < 0.001, respectively) but not for group C (NS; P = 0.22). The volume of dilution exerted a significant effect on dPItoe over time (P < 0.001); this is well illustrated by the marked difference between groups A and B vs. group C in Fig. 3. While the effect of dose on dPItoe over time was also statistically significant, (P = 0.04), visual comparison between groups A and B suggest that this was a modest effect. The dPItoe at 20 min for groups A, B, and C was equivalent to a 280%, 303%, and 59% change from baseline, respectively. Groups A and B were significantly different from group C (P = 0.006 and P = 0.01, respectively) but not significantly different from each other (P = 0.44).
There was a significant reduction in the pulse oximeter PI over time in the finger for group A (P = 0.015) but not for group B (NS; P = 0.09) or for group C (P = 0.20). Dose exerted a significant effect on dPIfinger over time (P = 0.02); this is well illustrated by comparing groups A vs. B and C in Fig. 3. Volume did not exert a significant effect on dPIfinger over time (P = 0.32). Pair-wise group comparisons did not reach statistical significance (group A vs. B; P = 0.14, group A vs. C; P = 0.08, group B vs. C; P = 1.0).
There was a significant increase in the temperature of the toe for groups A and B at 20 min (P = 0.05 and 0.04, respectively) but not for group C (NS; P = 0.28). Volume exerted a significant effect on dTtoe over time (P = 0.01), but there was no significant effect for dose (P = 0.13).
There was no significant change in the temperature of the finger over time for any group, and there was no significant change in the thigh-toe and the arm-finger temperature gradients over time for any group.
There was a significant change in the MAP over time for all groups (group A; P = 0.016, group B; P = 0.035, and group C; P = 0.011). The dMAP at 20 min was equivalent to a −12%, −6%, and a −7% change from baseline, respectively. There was no significant difference between groups.
Sensory and motor block
The level of sensory anesthesia to cold, touch, and pinprick increased over time in all groups. While there was a tendency for group A to exhibit slightly higher anesthetic levels in all sensory modalities tested, this was not significant (A vs. B; P = 0.22, A vs. C; P = 0.071, Fig. 4). There was no significant effect of the dose, volume, or concentration on the height of sensory block.
The number and percentage of patients in each group with intense motor block (Bromage 2 or 3) was higher in group A (n = 7, 54%) and group B (n = 7, 44%) than in group C (n = 2, 12%) and was affected by the volume of drug dilution (P < 0.001) rather than by absolute dose of drug (NS; P = 0.15) (Fig. 5).
Discussion
Bromage wrote that the spread, intensity, and analgesic effectiveness of epidural local anesthetics are dependent on the total dose administered, irrespective of its concentration or volume of dilution.23 Several studies have subsequently demonstrated identical sensory and motor blockade when using local anesthetic solutions of widely different concentrations but of equal drug dose.2–12,24,25 Some studies even demonstrated improved postoperative analgesia with concentrated epidural local anesthetic infusions9 and more profound motor block3,4 and hypotension3,4 when using dilute solutions. Conversely, other studies have been published that support the superiority of dilute epidural local anesthetics for diffuse low intensity analgesia, particularly in labor analgesia.1,10,26,27 While the cause for this division in the literature is not yet clear, it is likely that the clinical models chosen, the sensory modalities assessed, and the methods of assessment may impact on the relative effects of concentrated and dilute epidural local anesthetic solutions in these studies.
In the current study, we observed only a modest difference in the degree of sympathectomy when comparing epidural local anesthetics with different doses but with equal volumes of dilution. A far greater difference in sympathetic block was observed when comparing epidural local anesthetics with equal doses but with different volumes of dilution.
Interestingly, we observed no evidence for significant sympathectomy following the administration of 40 ml of 0.0625% bupivacaine, despite the presence of sensory levels to pinprick, cold, and light touch in almost all patients tested; and despite the typically excellent analgesia seen when using these doses in clinical practice. This suggests, at least in the doses and dilutions used in this study, that sensory neural function was more susceptible than sympathetic neural function to local anesthetic blockade. This is contrary to the classic teaching by Greene et al.28,29 50 years ago, who demonstrated differential sensory block following epidural anesthesia. Subsequently, the sympathetic level (assessed by thermography) was reported to be higher than the sensory level for pinprick by 6–7 dermatomes,30 and sympathectomy-mediated changes in skin blood flow were reported to occur earlier than sensory anesthesia to cold, pin-prick, and light touch by 4, 10, and 12 min, respectively.31 These studies contrast with a study (using both laser Doppler flowmetry and infrared thermography) which noted that the distribution of sympathetic-mediated vasodilatation was less extensive than sensory deficit following spinal anesthesia.32 These studies also contrast with a thermography study in patients with inadvertent total spinal anesthesia which observed vasoconstriction in the upper limb despite profound sensory and motor block of both arms.33 Like these latter reports, our observations are not consistent with the hypothesis that epidural local anesthetic blockade preferentially blocks sympathetic neural function more than sensory function. Indeed, the current use of dilute epidural local anesthetics to provide effective analgesia with minimal motor block or sympathectomy-related hemodynamic compromise suggests that epidural local anesthetics block sensation more readily than they block sympathetic neural function.
There are several limitations to this study. Out of the 60 cases, 14 were excluded from analysis because the recorded PPG waveforms did not meet a priori criteria for signal data quality. The typical mechanism for poor baseline PPG signal is poor contact between the patient and the probe. This is a common phenomenon occurring in 45% of intensive care patients34 and accounting for 77% of alarms from pulse oximeters in the post-anesthesia care unit.35 As algorithms for “on-line” quantitative PPG waveform analysis have only recently become available in new generation pulse oximeters (Masimo SET, Masimo Corporation, Irvine, CA, USA), waveforms were recorded without a real time waveform display and were subsequently analyzed “off-line,” by which time it was not possible to improve data signal quality. Although vascular disease and shock states may increase the likelihood of this phenomenon to some degree, both were a priori exclusion criteria in this study. Furthermore, there were no hemodynamic differences, age differences, or disease stratification differences between PPG accepted and PPG rejected patients. In addition, despite the vasomotor changes that were observed, no subject with acceptable baseline PPG data was subsequently rejected for poor PPG traces following the epidural. As a consequence, poor PPG signal in this study fits the criteria for “missing completely at random,”36 and the missing values would be expected to have the same distribution as the observed values. The potential for dropout bias was limited further by the blinded analysis of PPG waveforms and by a priori definitions of acceptable PPG signal quality. While the impact of the reduction in sample size may be relevant for comparisons that approached but did not reach significance, it is unlikely to have affected the primary outcome of this study which was designed with an initial power of 0.9.
We stopped measurements at 20 min following epidural analgesia to allow the ESWL procedure to proceed without excessive delay. We aimed to identify the onset of differential sympathetic, sensory, and motor block, rather than to determine the maximal changes. It is likely that we stopped data collection at a point prior to maximal expression of neural blockade.
We used both vasodilatation in the toe and compensatory vasoconstriction in the finger as indices of the extent and the intensity of sympathetic block. Compensatory vasoconstriction in the upper limb has been reported32,37 and reflects the effect of generalized sympathetic discharge on unblocked cervical or high thoracic sympathetic afferents and presumably contributes to the conservation of core temperature and arterial pressure.
This was not an electrophysiological study, and we do not make inferences regarding the susceptibilities of different sympathetic, sensory, and motor nerves to local anesthetic blockade. We have deliberately confined discussion to the effect on sympathetic, sensory, and motor function following epidural anesthesia.
In conclusion, within the range of doses tested and within the limitations of this study, our observations support the hypothesis that the extent and intensity of sympathectomy-mediated vasodilatation following lumbar epidural local anesthesia is predominantly dependent on the concentration of epidural local anesthetic rather than on the absolute dose of drug.
References
Christiaens F, Verborgh C, Dierick A, Camu F. Effects of diluent volume of a single dose of epidural bupivacaine in parturients during the first stage of labor. Reg Anesth Pain Med 1998; 23: 134–41.
Whiteside R, Jones D, Bignell S, Lang C, Lo SK. Epidural ropivacaine with fentanyl following major gynaecological surgery: the effect of volume and concentration on pain relief and motor impairment. Br J Anaesth 2000; 84: 720–4.
Dernedde M, Stadler M, Bardiau F, Boogaerts JG. Continuous epidural infusion of large concentration/small volume versus small concentration/large volume of levobupivacaine for postoperative analgesia. Anesth Analg 2003; 96: 796–801.
Dernedde M, Stadler M, Bardiau F, Boogaerts J. Comparison of different concentrations of levobupivacaine for post-operative epidural analgesia. Acta Anaesthesiol Scand 2003; 47: 884–90.
Duggan J, Bowler GM, McClure JH, Wildsmith JA. Extradural block with bupivacaine: influence of dose, volume, concentration and patient characteristics. Br J Anaesth 1988; 61: 324–31.
Galindo A, Benavides O, De Munos SO, Bonila O, Pena R. Comparison of anesthetic solutions used in lumbar and caudal peridural anesthesia. Anesth Analg 1978; 57: 175–9.
Laveaux MM, Hasenbos MA, Harbers JB, Liem T. Thoracic epidural bupivacaine plus sufentanil: high concentration/low volume versus low concentration/high volume. Reg Anesth 1993; 18: 39–43.
Liu SS, Ware PD, Rajendran S. Effects of concentration and volume of 2-chloroprocaine on epidural anesthesia in volunteers. Anesthesiology 1997; 86: 1288–93.
Murdoch JA, Dickson UK, Wilson PA, Berman JS, Gad-Elrab RR, Scott NB. The efficacy and safety of three concentrations of levobupivacaine administered as a continuous epidural infusion in patients undergoing orthopedic surgery. Anesth Analg 2002; 94: 438–44.
Nakayama M, Yamamoto J, Ichinose H, Yamamoto S, Kanaya N, Namiki A. Effects of volume and concentration of lidocaine on epidural anaesthesia in pregnant females. Eur J Anaesthesiol 2002; 19: 808–11.
Bernard JM, Le Roux D, Frouin J. Ropivacaine and fentanyl concentrations in patient-controlled epidural analgesia during labor: a volume-range study. Anesth Analg 2003; 97: 1800–7.
Chan L, Lee BB, Ngan Kee WD. A randomised double-blinded controlled trial of the effect of diluent volume on the efficacy of a single dose of epidural ropivacaine for labour analgesia. Int J Obst Anest 2006; 15: 201–5.
Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas 2007; 28: R1–39.
Reisner A, Shaltis PA, McCombie D, Asada HH. Utility of the photoplethysmogram in circulatory monitoring. Anesthesiology 2008; 108: 950–8.
Zaramella P, Freato F, Quaresima V, et al. Foot pulse oximeter perfusion index correlates with calf muscle perfusion measured by near infrared spectroscopy in healthy neonates. J Perinatol 2005; 25: 417–22.
Lima AP, Beelen P, Bakkar J. Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Crit Care Med 2002; 30: 1210–3.
Nasimi SG, Mearns AJ, Harness JB, Heath I. Quantitative measurement of sympathetic neuropathy in patients with diabetes mellitus. J Biomed Eng 1991; 13: 203–8.
Nitzan M, Babchenko A, Shemesh D, Alberton J. Influence of thoracic sympathectomy on cardiac induced oscillations in tissue blood volume. Med Biol Eng Comput 2001; 39: 579–83.
Babchenko A, Davidson E, Ginosar Y, et al. Photoplethysmographic measurement of changes in total and pulsatile tissue blood volume, following sympathetic blockade. Physiol Meas 2001; 22: 389–96.
Nijboer JA, Dorlas JC, Mahieu HF. Photoelectric plethysmography—some fundamental aspects of the reflection and transmission method. Clin Phys Physiol Meas 1981; 2: 205–15.
Nijboer JA, Dorlas JC. Comparison of plethysmograms taken from finger and pinna during anaesthesia. Br J Anaesth 1985; 57: 531–4.
Nitzan M, Babchenko A, Khanokh B, Landau D. The variability of the photoplethysmographic signal—a potential method for the evaluation of the autonomic nervous system. Physiol Meas 1998; 19: 93–102.
Bromage PR. Mechanism of action of extradural analgesia. Br J Anaesth 1975; 47(Suppl): 199–211.
Mogensen T, Scott NB, Hjortso NC, Lund C, Kehlet H. The influence of volume and concentration of bupivacaine on regression of analgesia during continuous postoperative epidural infusion. Reg Anesth 1988; 13: 122–5.
Noble HA, Enever GR, Thomas TA. Epidural bupivacaine dilution for labour. A comparison of three concentrations infused with a fixed dose of fentanyl. Anaesthesia 1991; 46: 549–52.
Snijdelaar DG, Hasenbos MA, van Egmond J, Wolff AP, Liem TH. High thoracic epidural sufentanil with bupivacaine: continuous infusion of high volume versus low volume. Anesth Analg 1994; 78: 490–4.
Okutomi T, Minakawa M, Hoka S. Saline volume and local anesthetic concentration modify the spread of epidural anesthesia. Can J Anesth 1999; 46: 930–4.
Greene NM. Area of differential block in spinal anesthesia with hyperbaric tetracaine. Anesthesiology 1958; 19: 45–50.
Brull SJ, Greene NM. Zones of differential sensory block during extradural anaesthesia. Br J Anaesth 1991; 66: 651–5.
Chamberlain DP, Chamberlain BD. Changes in the skin temperature of the trunk and their relationship to sympathetic blockade during spinal anesthesia. Anesthesiology 1986; 65: 139–43.
Valley MA, Bourke DL, Hamill MP, Raja SN. Time course of sympathetic blockade during epidural anesthesia: laser Doppler flowmetry studies of regional skin perfusion. Anesth Analg 1993; 76: 289–94.
Bengtsson M. Changes in skin blood flow and temperature during spinal analgesia evaluated by laser Doppler flowmetry and infrared thermography. Acta Anaesthesiol Scand 1984; 28: 625–30.
Kimura T, Goda Y, Kemmotsu O, Shimada Y. Regional differences in skin blood flow and temperature during total spinal anaesthesia. Can J Anaesth 1992; 39: 123–7.
Chambrin MC, Ravaux P, Calvelo-Aros D, Jaborska A, Chopin C, Boniface B. Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med 1999; 25: 1360–6.
Wiklund L, Hok B, Stahl K, Jordeby-Jonsson A. Postanesthesia monitoring revisited: frequency of true and false alarms from different monitoring devices. J Clin Anesth 1994; 6: 182–8.
Everitt BS. Analysis of longitudinal data. Beyond MANOVA. Br J Psychiatry 1998; 172: 7–10.
Matsukawa T, Sessler DI, Christensen R, Ozaki M, Schroeder M. Heat flow and distribution during epidural anesthesia. Anesthesiology 1995; 83: 961–7.
Acknowledgement
Funding: Chief Scientist Research Fund of Israel Ministry of Science and Technology (to M.N.) and E.W. Joseph fund (to M.N.). Clinical trial registry: This study is registered with Clinicaltrials.gov, the Protocol Registration System of the FDA and the NIH (ref number: NCT00197353).
Conflicts of interest
There are no conflicts of interest for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Study conducted in Department of Anesthesiology and Critical Care Medicine, Hadassah Hebrew University Medical Center, Jerusalem, Israel.
An erratum to this article can be found online at http://dx.doi.org/10.1007/s12630-010-9295-3.
Rights and permissions
About this article
Cite this article
Ginosar, Y., Weiniger, C.F., Kurz, V. et al. Sympathectomy-mediated vasodilatation: a randomized concentration ranging study of epidural bupivacaine. Can J Anesth/J Can Anesth 56, 213–221 (2009). https://doi.org/10.1007/s12630-008-9036-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-008-9036-z