Abstract
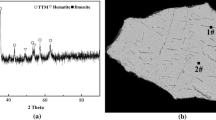
The isothermal reduction of the Panzhihua titanomagnetite concentrates (PTC) briquette containing coal under argon atmosphere was investigated by thermogravimetry in an electric resistance furnace within the temperature range of 1250–1350°C. The samples reduced in argon at 1350°C for different time were examined by X-ray diffraction (XRD) analysis. Model-fitting and model-free methods were used to evaluate the apparent activation energy of the reduction reaction. It is found that the reduction rate is very fast at the early stage, and then, at a later stage, the reduction rate becomes slow and decreases gradually to the end of the reduction. It is also observed that the reduction of PTC by coal depends greatly on the temperature. At high temperatures, the reduction degree reaches high values faster and the final value achieved is higher than at low temperatures. The final phase composition of the reduced PTC-coal briquette consists in iron and ferrous-pseudobrookite (FeTi2O5), while Fe2.75Ti0.25O4, Fe2.5Ti0.5O4, Fe2.25Ti0.75O4, ilmenite (FeTiO3) and wustite (FeO) are intermediate products. The reaction rate is controlled by the phase boundary reaction for reduction degree less than 0.2 with an apparent activation energy of about 68 kJ·mol−1 and by three-dimensional diffusion for reduction degree greater than 0.75 with an apparent activation energy of about 134 kJ·mol−1. For the reduction degree in the range of 0.2–0.75, the reaction rate is under mixed control, and the activation energy increases with the increase of the reduction degree.
Similar content being viewed by others
References
D.S. Chen, L.N. Wang, T. Qi, and B. Song, Study on preoxidation of vanadium-bearing titanomagnetite concentrates, J. Hunan Univ. Sci. Technol. Nat. Sci., 26(2011), No. 3, p. 95.
Z.J. Liu, G.Q. Yang, Q.G. Xue, J.L. Zhang, and T.J. Yang, Research on direct reduction of coal-containing pellets of vanadic-titanomagnetite by rotary hearth furnace, Chin. J. Process Eng., 9(2009), No. 1, p. 51.
L.H. Zhou, D.P. Tao, M.X. Fang, F.H. Zeng, and X. Pu, Carbothermic reduction of V-Ti magnetite ore, Chin. J. Rare Met., 33(2009), No. 3, p. 406.
G.H. Zhang, Z. Yan, Y.J. Feng, M. Guo, X.D. Wang, L.F. Li, and G.Z. Zhou, Reduction kinetics of vanadic titanomagnetite in Panzhihua, J. Chin. Rare Earth Soc., 26(2008), p. 210.
X. Xue, Research on direct reduction of vanadic titanomagnetite, Iron Steel Vanadium Titanium, 28(2007), No. 3, p. 37.
Y.Z. Lan and C.P. Liu, Kinetics of carbon catalytic reduction of titanomagnetite concentrate, Acta Metall. Sin., 32(1996), No. 5, p. 502.
E. Park and O. Ostrovski, Reduction of titania-ferrous ore by hydrogen, ISIJ Int., 44(2004), No. 6, p. 999.
E. Park and O. Ostrovski, Reduction of titania-ferrous ore by carbon monoxide, ISIJ Int., 43(2003), No. 9, p. 1316.
T. Hu, X.W. Lv, C.G. Bai, Z.G. Lun, and G.B. Qiu, Reduction behavior of Panzhihua titanomagnetite concentrates with coal, Metall. Mater. Trans. B, 44(2013), No. 2, p. 252.
X.W. Lv, Z.G. Lun, J.Q. Yin, and C.G. Bai, Carbothermic reduction of vanadium titanomagnetite by microwave irradiation and smelting behavior, ISIJ Int., 53(2013), No. 7, p. 1115.
H.Y. Sun, X.J. Dong, X.F. She, Q.G. Xue, and J.S. Wang, Solid state reduction of titanomagnetite concentrate by graphite, ISIJ Int., 53(2013), No. 4, p. 564.
H.Y. Sun, J.S. Wang, Y.H. Han, X.F. She, and Q.G. Xue, Reduction mechanism of titanomagnetite concentrate by hydrogen, Int. J. Miner. Process., 125(2013), No. 12, p. 122.
J.L. Zhang, X.D. Xing, M.M. Cao, K.X. Jiao, C.L. Wang, and S. Ren, Reduction kinetics of vanadium titano-magnetite carbon composite pellets adding catalysts under high temperature, J. Iron Steel Res. Int., 20(2013), No. 2, p. 1.
S.K. Dey, B. Jana, and A. Basumallick, Kinetics and reduction characteristics of hematite-noncoking coal mixed pellets under nitrogen gas atmosphere, ISIJ Int., 33(1993), No. 7, p. 735.
S. Vyazovkin and C.A. Wight, Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data, Thermochim. Acta, 340-341(1999), p. 53.
M.E. Brown, D. Dollimore, and A.K. Galwey, Reactions in the solid state, [in] Comprehensive Chemical Kinetics, Elsevier, 22(1980), p. 347.
J.H. Sharp, G.W. Brindley, and B.N. Narahari, Numerical data for some commonly used solid state reaction equations, J. Am. Ceram. Soc., 49(1966), No. 7, p. 379.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, T., Lü, Xw., Bai, Cg. et al. Isothermal reduction of titanomagnetite concentrates containing coal. Int J Miner Metall Mater 21, 131–137 (2014). https://doi.org/10.1007/s12613-014-0875-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-014-0875-z